State of the art direct 13C and indirect 1H-[13C] NMR spectroscopy in vivo. A practical guide
- PMID: 21919099
- PMCID: PMC3694136
- DOI: 10.1002/nbm.1761
State of the art direct 13C and indirect 1H-[13C] NMR spectroscopy in vivo. A practical guide
Abstract
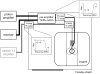
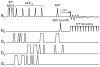
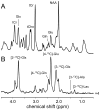
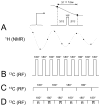
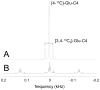
Carbon-13 NMR spectroscopy in combination with (13)C-labeled substrate infusion is a powerful technique for measuring a large number of metabolic fluxes noninvasively in vivo. It has been used to quantify glycogen synthesis rates, establish quantitative relationships between energy metabolism and neurotransmission, and evaluate the importance of different substrates. Measurements can, in principle, be performed through direct (13)C NMR detection or via indirect (1)H-[(13)C] NMR detection of the protons attached to (13)C nuclei. The choice of detection scheme and pulse sequence depends on the magnetic field strength, whereas substrate selection depends on metabolic pathways. (13)C NMR spectroscopy remains a challenging technique that requires several nonstandard hardware modifications, infusion of (13)C-labeled substrates, and sophisticated processing and metabolic modeling. In this study, the various aspects of direct (13)C and indirect (1)H-[(13)C] NMR are reviewed with the aim of providing a practical guide.
Copyright © 2011 John Wiley & Sons, Ltd.
Figures




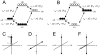
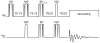

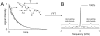

References
-
- Siesjo B. Brain energy metabolism. John Wiley; New York: 1978.
-
- Raichle ME, Larson KB, Phelps ME, Grubb RL, Jr, Welch MJ, Ter-Pogossian MM. In vivo measurement of brain glucose transport and metabolism employing glucose-11C. Am J Physiol. 1975;228:1936–1948. - PubMed
-
- Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916. - PubMed
-
- Phelps ME, Huang SC, Hoffman EJ, Selin C, Sokoloff L, Kuhl DE. Tomographic measurement of local cerebral glucose metabolic rate in humans with 18F-2-fluoro-2-deoxy-D-glucose: validation of method. Ann Neurol. 1979;6:371–388. - PubMed
-
- Reivich M, Kuhl D, Wolf A, Greenberg J, Phelps M, Ido T, Casella V, Fowler J, Hoffman E, Alavi A, Som P, Sokoloff L. The [18F]fluorodeoxyglucose method for the measurement of local cerebral glucose utilization in man. Circ Res. 1979;44:127–137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

