Dissociation between the processivity and total activity of γ-secretase: implications for the mechanism of Alzheimer's disease-causing presenilin mutations
- PMID: 21919498
- PMCID: PMC3205908
- DOI: 10.1021/bi2007146
Dissociation between the processivity and total activity of γ-secretase: implications for the mechanism of Alzheimer's disease-causing presenilin mutations
Abstract
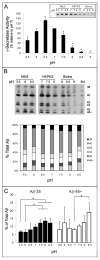
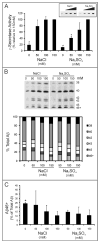
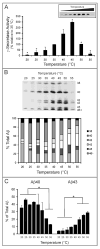
The amyloid β-peptide (Aβ), strongly implicated in the pathogenesis of Alzheimer's disease (AD), is produced from the amyloid β-protein precursor (APP) through consecutive proteolysis by β- and γ-secretases. The latter protease contains presenilin as the catalytic component of a membrane-embedded aspartyl protease complex. Missense mutations in presenilin are associated with early-onset familial AD, and these mutations generally both decrease Aβ production and increase the ratio of the aggregation-prone 42-residue form (Aβ42) to the 40-residue form (Aβ40). The connection between these two effects is not understood. Besides Aβ40 and Aβ42, γ-secretase produces a range of Aβ peptides, the result of initial cutting at the ε site to form Aβ48 or Aβ49 and subsequent trimming every three or four residues. Thus, γ-secretase displays both overall proteolytic activity (ε cutting) and processivity (trimming) toward its substrate APP. Here we tested whether a decrease in total activity correlates with decreased processivity using wild-type and AD-mutant presenilin-containing protease complexes. Changes in pH, temperature, and salt concentration that reduced the overall activity of the wild-type enzyme did not consistently result in increased proportions of longer Aβ peptides. Low salt concentrations and acidic pH were notable exceptions that subtly alter the proportion of individual Aβ peptides, suggesting that the charged state of certain residues may influence processivity. Five different AD mutant complexes, representing a broad range of effects on overall activity, Aβ42:Aβ40 ratios, and ages of disease onset, were also tested, revealing again that changes in total activity and processivity can be dissociated. Factors that control initial proteolysis of APP at the ε site apparently differ significantly from factors affecting subsequent trimming and the distribution of Aβ peptides.
Figures



References
-
- Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Comm. 1984;120:885–890. - PubMed
-
- Wolfe MS. The gamma-secretase complex: membrane-embedded proteolytic ensemble. Biochemistry. 2006;45:7931–7939. - PubMed
-
- Jarrett JT, Berger EP, Lansbury PT., Jr The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer’s disease. Biochemistry. 1993;32:4693–4697. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

