Response of a preosteoblastic cell line to cyclic tensile stress conditioning and growth factors for bone tissue engineering
- PMID: 21919794
- PMCID: PMC3267964
- DOI: 10.1089/ten.TEA.2010.0414
Response of a preosteoblastic cell line to cyclic tensile stress conditioning and growth factors for bone tissue engineering
Abstract
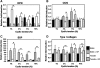
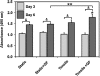
Bone regeneration can be accelerated by utilizing mechanical stress and growth factors (GFs). However, a limited understanding exists regarding the response of preosteoblasts to tensile stress alone or with GFs. We measured cell proliferation and expression of heat-shock proteins (HSPs) and other bone-related proteins by preosteoblasts following cyclic tensile stress (1%-10% magnitude) alone or in combination with bone morphogenetic protein-2 (BMP-2) and transforming growth factor-β1 (TGF-β1). Tensile stress (3%) with GFs induced greater gene upregulation of osteoprotegerin (3.3 relative fold induction [RFI] compared to sham-treated samples), prostaglandin E synthase 2 (2.1 RFI), and vascular endothelial growth factor (VEGF) (11.5 RFI), compared with samples treated with stimuli alone or sham-treated samples. The most significant increases in messenger RNA expression occurred with GF addition to either static-cultured or tensile-loaded (1% elongation) cells for the following genes: HSP47 (RFI=2.53), cyclooxygenase-2 (RFI=72.52), bone sialoprotein (RFI=11.56), and TGF-β1 (RFI=8.05). Following 5% strain with GFs, VEGF secretion increased 64% (days 3-6) compared with GF alone and cell proliferation increased 23% compared with the sham-treated group. GF addition increased osteocalcin secretion but decreased matrix metalloproteinase-9 significantly (days 3-6). Tensile stress and GFs in combination may enhance bone regeneration by initiating angiogenic and anti-osteoclastic effects and promote cell growth.
Figures



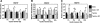


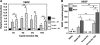

Similar articles
-
Influence of heating and cyclic tension on the induction of heat shock proteins and bone-related proteins by MC3T3-E1 cells.Biomed Res Int. 2014;2014:354260. doi: 10.1155/2014/354260. Epub 2014 Jun 11. Biomed Res Int. 2014. PMID: 25013774 Free PMC article.
-
Response of preosteoblasts to thermal stress conditioning and osteoinductive growth factors.Cell Stress Chaperones. 2012 Mar;17(2):203-14. doi: 10.1007/s12192-011-0300-8. Epub 2011 Nov 25. Cell Stress Chaperones. 2012. PMID: 22116637 Free PMC article.
-
Experimental study of the synergistic effect and network regulation mechanisms of an applied combination of BMP-2, VEGF, and TGF-β1 on osteogenic differentiation.J Cell Biochem. 2020 Mar;121(3):2394-2405. doi: 10.1002/jcb.29462. Epub 2019 Oct 23. J Cell Biochem. 2020. PMID: 31646676
-
The sequential production profiles of growth factors and their relations to bone volume in ossifying bone marrow explants.Tissue Eng Part A. 2010 Jul;16(7):2295-306. doi: 10.1089/ten.TEA.2009.0565. Tissue Eng Part A. 2010. PMID: 20184436
-
Enhancement of bone morphogenetic protein-2-induced ectopic bone formation by transforming growth factor-β1.Tissue Eng Part A. 2011 Mar;17(5-6):597-606. doi: 10.1089/ten.TEA.2010.0094. Epub 2010 Nov 9. Tissue Eng Part A. 2011. PMID: 20874259
Cited by
-
Effects of vascular endothelial growth factor 165 on bone tissue engineering.PLoS One. 2013 Dec 20;8(12):e82945. doi: 10.1371/journal.pone.0082945. eCollection 2013. PLoS One. 2013. PMID: 24376611 Free PMC article.
-
Serum exosomes from young rats improve the reduced osteogenic differentiation of BMSCs in aged rats with osteoporosis after fatigue loading in vivo.Stem Cell Res Ther. 2021 Jul 27;12(1):424. doi: 10.1186/s13287-021-02449-9. Stem Cell Res Ther. 2021. PMID: 34315544 Free PMC article.
-
Influence of heating and cyclic tension on the induction of heat shock proteins and bone-related proteins by MC3T3-E1 cells.Biomed Res Int. 2014;2014:354260. doi: 10.1155/2014/354260. Epub 2014 Jun 11. Biomed Res Int. 2014. PMID: 25013774 Free PMC article.
-
Liver-targeting iron oxide nanoparticles and their complexes with plant extracts for biocompatibility.Beilstein J Nanotechnol. 2024 Dec 11;15:1593-1602. doi: 10.3762/bjnano.15.125. eCollection 2024. Beilstein J Nanotechnol. 2024. PMID: 39691205 Free PMC article.
-
Symptomatic stress reaction of the humerus in a professional cricketer.BMJ Case Rep. 2019 Sep 18;12(9):e227088. doi: 10.1136/bcr-2018-227088. BMJ Case Rep. 2019. PMID: 31537584 Free PMC article.
References
-
- Bhatt K.A. Chang E.I. Warren S.M. Lin S.E. Bastidas N. Ghali S., et al. Uniaxial mechanical strain: an in vitro correlate to distraction osteogenesis. J Surg Res. 2007;143:329. - PubMed
-
- Kanno T. Takahashi T. Tsujisawa T. Ariyoshi W. Nishihara T. Mechanical stress-mediated Runx2 activation is dependent on Ras/ERK1/2 MAPK signaling in osteoblasts. J Cell Biochem. 2007;101:1266. - PubMed
-
- Singh S.P. Chang E.I. Gossain A.K. Mehara B.J. Galiano R.D. Jensen J., et al. Cyclic mechanical strain increases production of regulators of bone healing in cultured murine osteoblasts. J Am Coll Surg. 2007;204:426. - PubMed
-
- Garvin J. Qi J. Maloney M. Banes A.J. Novel system for engineering bioartificial tendons and application of mechanical load. Tissue Eng. 2003;9:967. - PubMed
-
- Stegemann J.P. Nerem R.M. Phenotype modulation in vascular tissue engineering using biochemical and mechanical stimulation. Ann Biomed Eng. 2003;31:391. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

