Dual regulation of breast tubulogenesis using extracellular matrix composition and stromal cells
- PMID: 21919795
- PMCID: PMC3286825
- DOI: 10.1089/ten.TEA.2011.0317
Dual regulation of breast tubulogenesis using extracellular matrix composition and stromal cells
Abstract
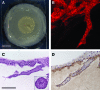
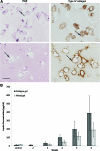
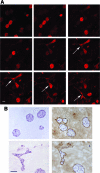
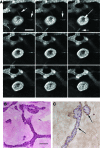
Epithelial-mesenchymal interactions during embryogenesis are critical in defining the phenotype of tissues and organs. The initial elongation of the mammary bud represents a central morphological event requiring extensive epithelial-mesenchymal crosstalk. The precise mechanism orchestrating this outgrowth is still unknown and mostly animal models have been relied upon to explore this process. Highly tunable three-dimensional (3D) culture models are a complementary approach to address the question of phenotypic determination. Here, we used a 3D in vitro culture to study the roles of stromal cells and extracellular matrix components during mammary tubulogenesis. Fibroblasts, adipocytes, and type I collagen actively participated in this process, whereas reconstituted basement membrane inhibited tubulogenesis by affecting collagen organization. We conclude that biochemical and biomechanical signals mediate the interaction between cells and matrix components and are necessary to induce tubulogenesis in vitro.
Figures





References
-
- Kratochwil K. Organ specificity in mesenchymal induction demonstrated in the embryonic development of the mammary gland of the mouse. Dev Biol. 1969;20:46. - PubMed
-
- Montesano R. Soriano J.V. Pepper M.S. Orci L. Induction of epithelial branching tubulogenesis in vitro. J Cell Physiol. 1997;173:152. - PubMed
-
- Sakakura T. Nishizuka Y. Dawe C.J. Mesenchyme-dependent morphogenesis and epithelium-specific cytodifferentiation in mouse mammary gland. Science. 1976;194:1439. - PubMed
-
- Cunha G.R. Role of mesenchymal-epithelial interactions in normal and abnormal development of the mammary gland and prostate. Cancer. 1994;74:1030. - PubMed
-
- Zangani D. Darcy K.M. Shoemaker S. Ip M.M. Adipocyte-epithelial interactions regulate the in vitro development of normal mammary epithelial cells. Exp Cell Res. 1999;247:399. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

