Tissue engineering a fetal membrane
- PMID: 21919796
- PMCID: PMC3267961
- DOI: 10.1089/ten.TEA.2011.0194
Tissue engineering a fetal membrane
Abstract
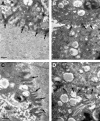
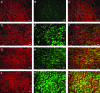
The aim of this study was to construct an artificial fetal membrane (FM) by combination of human amniotic epithelial stem cells (hAESCs) and a mechanically enhanced collagen scaffold containing encapsulated human amniotic stromal fibroblasts (hASFs). Such a tissue-engineered FM may have the potential to plug structural defects in the amniotic sac after antenatal interventions, or to prevent preterm premature rupture of the FM. The hAESCs and hASFs were isolated from human fetal amniotic membrane (AM). Magnetic cell sorting was used to enrich the hAESCs by positive ATP-binding cassette G2 selection. We investigated the use of a laminin/fibronectin (1:1)-coated compressed collagen gel as a novel scaffold to support the growth of hAESCs. A type I collagen gel was dehydrated to form a material mimicking the mechanical properties and ultra-structure of human AM. hAESCs successfully adhered to and formed a monolayer upon the biomimetic collagen scaffold. The resulting artificial membrane shared a high degree of similarity in cell morphology, protein expression profiles, and structure to normal fetal AM. This study provides the first line of evidence that a compacted collagen gel containing hASFs could adequately support hAESCs adhesion and differentiation to a degree that is comparable to the normal human fetal AM in terms of structure and maintenance of cell phenotype.
Figures

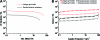




Similar articles
-
Ex vivo construction of an artificial ocular surface by combination of corneal limbal epithelial cells and a compressed collagen scaffold containing keratocytes.Tissue Eng Part A. 2010 Jun;16(6):2091-100. doi: 10.1089/ten.TEA.2009.0748. Tissue Eng Part A. 2010. PMID: 20109018
-
Reconstruction of a human hemicornea through natural scaffolds compatible with the growth of corneal epithelial stem cells and stromal keratocytes.Mol Vis. 2009 Oct 17;15:2084-93. Mol Vis. 2009. PMID: 19862337 Free PMC article.
-
The Role of Prolactin in Amniotic Membrane Regeneration: Therapeutic Potential for Premature Rupture of Membranes.Endocrinology. 2024 Jul 26;165(9):bqae095. doi: 10.1210/endocr/bqae095. Endocrinology. 2024. PMID: 39082703
-
Human amniotic epithelial stem cells, a potential therapeutic approach for diabetes and its related complications.Hum Cell. 2025 Jan 3;38(2):39. doi: 10.1007/s13577-024-01171-x. Hum Cell. 2025. PMID: 39753919 Review.
-
Stem cells from fetal membranes - a workshop report.Placenta. 2008 Mar;29 Suppl A:S17-9. doi: 10.1016/j.placenta.2007.11.006. Epub 2007 Dec 21. Placenta. 2008. PMID: 18155293
Cited by
-
Stem cells and COVID-19: are the human amniotic cells a new hope for therapies against the SARS-CoV-2 virus?Stem Cell Res Ther. 2021 Mar 1;12(1):155. doi: 10.1186/s13287-021-02216-w. Stem Cell Res Ther. 2021. PMID: 33648582 Free PMC article. Review.
-
Tissuepatch is biocompatible and seals iatrogenic membrane defects in a rabbit model.Prenat Diagn. 2018 Jan;38(2):99-105. doi: 10.1002/pd.5191. Epub 2017 Dec 11. Prenat Diagn. 2018. PMID: 29178347 Free PMC article.
-
Novel approach to gastric mucosal defect repair using fresh amniotic membrane allograft in dogs (experimental study).Stem Cell Res Ther. 2017 Oct 18;8(1):235. doi: 10.1186/s13287-017-0682-3. Stem Cell Res Ther. 2017. PMID: 29047409 Free PMC article.
-
Successful pregnancy outcome after septum resection and use of amnion graft in patient with high transverse vaginal septum.BMJ Case Rep. 2019 Jun 16;12(6):e228769. doi: 10.1136/bcr-2018-228769. BMJ Case Rep. 2019. PMID: 31208981 Free PMC article.
-
Comparison of human amniotic membrane decellularisation approaches for hESC-derived RPE cells culture.BMJ Open Ophthalmol. 2022 Sep;7(1):e000981. doi: 10.1136/bmjophth-2022-000981. BMJ Open Ophthalmol. 2022. PMID: 36161850 Free PMC article.
References
-
- Penny C.M. Stephen C.B. The fetal membranes and mechanisms underlying their labour-associated and pre-labour rupture during pregnancy. Fetal Matern Med Rev. 2004;15:73.
-
- Weisz B. David A.L. Chitty L. Peebles D. Pandya P. Patel P. Rodeck C.K. Association of isolated short femur in the mid-trimester fetus with perinatal outcome. Ultrasound Obstet Gynaecol. 2008;31:512. - PubMed
-
- Chandiramani M. Shennan A.H. Preterm labour: update on prediction and prevention strategies. Curr Opin Obstet Gynecol. 2006;18:618. - PubMed
-
- Deprest J. Lerut T.E. Vandenberghe K. Operative fetoscopy: new perspective in fetal therapy? Prenat Diagn. 1997;17:1247. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

