Comprehensive analysis of tumor microenvironment cytokines in Waldenstrom macroglobulinemia identifies CCL5 as a novel modulator of IL-6 activity
- PMID: 21921047
- PMCID: PMC3217355
- DOI: 10.1182/blood-2011-04-351742
Comprehensive analysis of tumor microenvironment cytokines in Waldenstrom macroglobulinemia identifies CCL5 as a novel modulator of IL-6 activity
Abstract
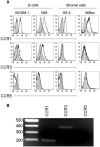
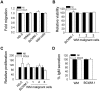
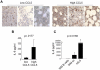
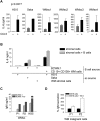
Although proinflammatory and chemotactic cytokines can profoundly affect the tumor microenvironment, and many of them have been shown to have therapeutic efficacy in preclinical models, the role of these molecules in Waldenström macroglobulinemia (WM) remains poorly understood. In this study, simultaneous analysis of WM patient sera and bone marrow biopsies identified a set of dysregulated cytokines including CCL5, G-CSF, and soluble IL-2 receptor, that were significantly elevated in WM patients whereas IL-8 and EGF levels were significantly lower in these patients compared with healthy controls. Interestingly, CCL5 levels positively correlated with features of disease aggressiveness such as elevated IgM levels and bone marrow involvement. Functional analysis of tumor microenvironment revealed a functional correlation between CCL5 levels and IL-6 levels, a proinflammatory cytokine with an important role in normal and malignant B-cell biology. Furthermore, CCL5 stimulated IL-6 secretion in WM stromal cells resulting in increased IgM secretion by WM malignant cells via the JAK/STAT signaling pathway. Thus, together these results define a novel signaling network in the WM tumor microenvironment controlling IgM secretion and suggest CCL5 as a potential target for the treatment of this disease.
Figures



References
-
- Grivennikov SI, Karin M. Inflammatory cytokines in cancer: tumour necrosis factor and interleukin 6 take the stage. Ann Rheum Dis. 2011;70(suppl 1):i104–108. - PubMed
-
- Goldstein H, Kim A. Immunoglobulin secretion and phosphorylation of common proteins are induced by IL-2, IL-4, and IL-6 in the factor responsive human B cell line, SKW6.4. J Immunol. 1993;151(12):6701–6711. - PubMed
-
- Weidle UH, Klostermann S, Eggle D, Kruger A. Interleukin 6/interleukin 6 receptor interaction and its role as a therapeutic target for treatment of cachexia and cancer. Cancer Genomics Proteomics. 2010;7(6):287–302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

