Hypoxia is present in murine atherosclerotic plaques and has multiple adverse effects on macrophage lipid metabolism
- PMID: 21921268
- PMCID: PMC3208906
- DOI: 10.1161/CIRCRESAHA.111.246363
Hypoxia is present in murine atherosclerotic plaques and has multiple adverse effects on macrophage lipid metabolism
Abstract
Rationale: Human atherosclerotic plaques contain large numbers of cells deprived of O(2). In murine atherosclerosis, because the plaques are small, it is controversial whether hypoxia can occur.
Objective: To examine if murine plaques contain hypoxic cells, and whether hypoxia regulates changes in cellular lipid metabolism and gene expression in macrophages.
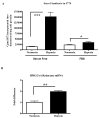
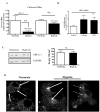
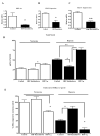
Methods and results: Aortic plaques from apolipoprotein-E-deficient mice were immunopositive for hypoxia-inducible transcription factor (HIF-1α) and some of its downstream targets. Murine J774 macrophages rendered hypoxic demonstrated significant increases in cellular sterol and triglycerides. The increase in sterol content in hypoxic macrophages correlated with elevated 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase activity and mRNA levels. In addition, when macrophages were incubated with cholesterol complexes, hypoxic cells accumulated 120% more cholesterol, predominately in the free form. Cholesterol-efflux assays showed that hypoxia significantly decreased efflux mediated by ATP-binding cassette subfamily A member 1 (ABCA1), whose sub cellular localization was altered in both J774 and primary macrophages. Furthermore, in vivo expression patterns of selected genes from cells in hypoxic regions of murine plaques were similar to those from J774 and primary macrophages incubated in hypoxia. The hypoxia-induced accumulation of sterol and decreased cholesterol efflux was substantially reversed in vitro by reducing the expression of the hypoxia-inducible transcription factor, HIF-1α.
Conclusion: Hypoxic regions are present in murine plaques. Hypoxic macrophages have increased sterol content due to the induction of sterol synthesis and the suppression of cholesterol efflux, effects that are in part mediated by HIF-1α.
Figures





References
-
- Davidson MH. Overview of prevention and treatment of atherosclerosis with lipid-altering therapy for pharmacy directors. Am J Manag Care. 2007;13(Suppl 10):S260–269. - PubMed
-
- Mensah GA, Brown DW. An overview of cardiovascular disease burden in the united states. Health Aff (Millwood) 2007;26:38–48. - PubMed
-
- Levin M, Leppanen O, Evaldsson M, Wiklund O, Bondjers G, Bjornheden T. Mapping of atp, glucose, glycogen, and lactate concentrations within the arterial wall. Arterioscler Thromb Vasc Biol. 2003;23:1801–1807. - PubMed
-
- Bjornheden T, Levin M, Evaldsson M, Wiklund O. Evidence of hypoxic areas within the arterial wall in vivo. Arterioscler Thromb Vasc Biol. 1999;19:870–876. - PubMed
-
- Leineweber C, Kecklund G, Janszky I, Akerstedt T, Orth-Gomer K. Snoring and progression of coronary artery disease: The stockholm female coronary angiography study. Sleep. 2004;27:1344–1349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

