Concurrent vs sequential adjuvant chemotherapy and hormone therapy in breast cancer: a multicenter randomized phase III trial
- PMID: 21921285
- PMCID: PMC3202939
- DOI: 10.1093/jnci/djr351
Concurrent vs sequential adjuvant chemotherapy and hormone therapy in breast cancer: a multicenter randomized phase III trial
Abstract
Background: The most appropriate timing of chemotherapy and hormone therapy administration is a critical issue in early breast cancer patients. The purpose of our study was to compare the efficacy of concurrent vs sequential administration of adjuvant chemotherapy and tamoxifen.
Methods: Women with node-positive primary breast cancer were randomly assigned to receive tamoxifen (20 mg/d for 5 years) during (concurrent arm) or after (sequential arm) adjuvant chemotherapy. Chemotherapy consisted of alternating regimens of cyclophosphamide, epidoxorubicin, and 5-fluorouracil and cyclophosphamide, methotrexate, and 5-fluorouracil every 21 days for a total of 12 cycles. The primary endpoint was overall survival (OS), and secondary endpoints were toxic effects and disease-free survival (DFS). No provision for interim analyses was made in the original study protocol. Survival curves were estimated by the Kaplan-Meier method. Multivariable Cox regression models, adjusted for age, menopausal status, tumor stage, and lymph node and hormone receptor status, were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). All statistical tests were two-sided.
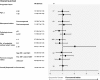
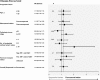
Results: From 1985 to 1992, 431 patients were randomly assigned and studied according to the intention-to-treat principle. After a maximum of 15.4 years of follow-up (median 12.3 years), the estimated actuarial 10-year OS was equivalent for the two study arms (concurrent arm: 111 patients, 66%, 95% CI = 59% to 72%; sequential arm: 114 patients, 65%, 95% CI = 59% to 72%, P = .86). No differences in DFS and toxic effects were evident. Four interim analyses were performed, but no alpha error adjustment was necessary because of the largely negative results of this final analysis (sequential vs concurrent arm: HR of death = 1.06, 95% CI = 0.78 to 1.44, P = .76; HR of relapse = 1.16, 95% CI = 0.88 to 1.52, P = .36).
Conclusions: No statistically significant differences in OS, DFS, and toxic effects between concurrent and sequential adjuvant chemo- and hormone therapies were observed. Our study does not support the superiority of one schedule of chemo- and hormone-therapy administration over the other. However, because of the limited statistical power of the study, these results must be considered with caution.
Figures

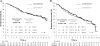
References
-
- Abe O, Abe R, Enomoto K, et al. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. - PubMed
-
- Sertoli MR, Scarsi PG, Rosso R. Rationale for combining chemotherapy and hormonal therapy in breast cancer. J Steroid Biochem. 1985;23(6B):1097–1103. - PubMed
-
- Osborne CK, Kitten L, Arteaga CL. Antagonism of chemotherapy-induced cytotoxicity for human breast cancer cells by antiestrogens. J Clin Oncol. 1989;7(6):710–717. - PubMed
-
- Hug V, Hortobagyi GB, Drewinko B, Finders M. Tamoxifen-citrate counteracts the antitumor effects of cytotoxic drugs in vitro. J Clin Oncol. 1985;3(12):1672–1677. - PubMed
-
- Osborne CK. Combined chemo-hormonal therapy in breast cancer: a hypothesis. Breast Cancer Res Treat. 1981;1(2):121–123. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

