Presence of preexisting antibodies mediates survival in sepsis
- PMID: 21921828
- PMCID: PMC3241849
- DOI: 10.1097/SHK.0b013e3182356f3e
Presence of preexisting antibodies mediates survival in sepsis
Abstract
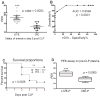
Sepsis is one of the leading causes of death in hospitals worldwide. Even with optimal therapy, severe sepsis results in 50% mortality, indicating variability in the response of individuals towards treatment. We hypothesize that the presence of preexisting antibodies present in the blood before the onset of sepsis induced by cecal ligation and puncture (CLP) in mice accounts for the differences in their survival. A plasma-enhanced killing (PEK) assay was performed to calculate the PEK capacity of plasma, that is, the ability of plasma to augment polymorphonuclear neutrophil killing of bacteria. Plasma-enhanced killing was calculated as PEK = [1 / log (N)] × 100, where N = number of surviving bacteria; a higher PEK indicated better bacterial killing. A range of PEK in plasma collected from mice before CLP was observed, documenting individual differences in bacterial killing capacity. Mortality was predicted based on plasma IL-6 levels at 24 h after CLP. Mice predicted to die (Die-P) had a lower PEK (<14) and higher peritoneal bacterial counts at 24 h after sepsis compared with those predicted to live (Live-P) with a PEK of greater than 16. Mice with PEK of less than 14 were 3.1 times more likely to die compared with the group with PEK of greater than 16. To understand the mechanism of defense conferred by the preexisting antibodies, binding of IgM or IgG to enteric bacteria was documented by flow cytometry. To determine the relative contribution of IgM or IgG, the immunoglobulins were specifically immunodepleted from the naive plasma samples and the PEK of the depleted plasma measured. Compared with naive plasma, depletion of IgM had no effect on the PEK. However, depletion of IgG increased PEK, suggesting that an inhibitory IgG binds to antigenic sites on bacteria preventing optimal opsonization of the bacteria. These data demonstrate that, before CLP, circulating inhibitory IgG antibodies exist that prevent bacterial killing by polymorphonuclear neutrophils in a CLP model of sepsis.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures





References
-
- Angus DC, Pereira CA, Silva E. Epidemiology of Severe Sepsis around the World. Endocr Metab Immune Disord Drug Targets. 2006;6(2):207–12. - PubMed
-
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of Severe Sepsis in the United States: Analysis of Incidence, Outcome, and Associated Costs of Care. Crit Care Med. 2001;29(7):1303–10. - PubMed
-
- Angus DC, Wax RS. Epidemiology of Sepsis: An Update. Crit Care Med. 2001;29(7 Suppl):S109–16. - PubMed
-
- Quartin AA, Schein RM, Kett DH, Peduzzi PN. Magnitude and Duration of the Effect of Sepsis on Survival. Department of Veterans Affairs Systemic Sepsis Cooperative Studies Group. Jama. 1997;277(13):1058–63. - PubMed
-
- Martin GS, Mannino DM, Eaton S, Moss M. The Epidemiology of Sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

