Autosomal dominant Best disease with an unusual electrooculographic light rise and risk of angle-closure glaucoma: a clinical and molecular genetic study
- PMID: 21921978
- PMCID: PMC3171497
Autosomal dominant Best disease with an unusual electrooculographic light rise and risk of angle-closure glaucoma: a clinical and molecular genetic study
Abstract
Purpose: To describe the clinical and molecular characteristics of two families with autosomal dominant Best disease and atypical electrooculography (EOG).
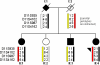
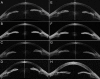
Methods: Four affected individuals from two families were ascertained. Detailed ophthalmic examinations, refraction, and biometry (anterior chamber depth [ACD] and axial length [AL]), gonioscopy, optical coherence tomography of the anterior segment and retina, retinal imaging, and electrophysiological assessment were performed. Arden ratios from EOG testing were calculated by direct measurement of the light peak to dark trough amplitudes. Mutations in bestrophin 1 (BEST1) were identified by bidirectional Sanger sequencing. In family 1, segregation of BEST1 alleles was performed by assaying four microsatellite markers (D11S935, D11S4102, D11S987, and D11S4162) that flank BEST1.
Results: The proband from family 1 (three of four siblings affected with Best disease) was 42 years old with bilateral macular vitelliform lesions, advanced angle closure glaucoma (ACG), a normal electroretinogram, and no EOG light rise. Her 44-year-old brother had similar fundus appearances and an EOG light rise of 170%. Their 48-year-old sister had a normal left fundus, whereas the right fundus showed a vitelliform lesion and subretinal thickening. There was no EOG light rise detectable from either eye. Mutation analysis of BEST1 showed all affected siblings to be heterozygous for a missense mutation, c.914T>C, p.Phe305Ser. Their unaffected sister had an EOG light rise of 200%, a normal fundus appearance, and did not harbor the BEST1 mutation. Haplotype analysis of family 1 showed that the affected brother with the 170% EOG light rise had inherited the same nondiseased parental BEST1 allele as his unaffected sister. The other two affected sisters with undetectable EOG light rises shared a different nondiseased parental BEST1 allele. An unrelated 53-year-old female carrying the same c.914T>C, p.Phe305Ser mutation showed typical features of Best disease and an EOG light rise of 180%. All four siblings from family 1 had shorter axial biometry (ACD range 2.06-2.74 mm; AL range 20.46-22.60 mm) than the normal population, contributing to their risk of ACG development. Proband 2 had deeper ACDs (2.83 mm OD and 2.85 mm OS), but similar ALs (21.52 mm OD and 21.42 mm OS) compared to family 1. She had no gonioscopic evidence of angle closure.
Conclusions: A near normal EOG light rise is uncommon in molecularly confirmed Best disease, and in the present report is associated with the same mutation in two families, suggesting a specific role for this amino acid in the retinal pigment epithelium dysfunction associated with this disorder. Haplotype analysis in family 1 was consistent with an effect of the nondisease allele in mediating the presence of an EOG light rise. Clinical assessment of ACG risk is recommended for BEST1 mutation carriers and their first degree relatives.
Figures




References
-
- Marquardt A, Stohr H, Passmore LA, Kramer F, Rivera A, Weber BH. Mutations in a novel gene, VMD2, encoding a protein of unknown properties cause juvenile-onset vitelliform macular dystrophy (Best's disease). Hum Mol Genet. 1998;7:1517–25. - PubMed
-
- Petrukhin K, Koisti MJ, Bakall B, Li W, Xie G, Marknell T, Sandgren O, Forsman K, Holmgren G, Andreasson S, Vujic M, Bergen AA, McGarty-Dugan V, Figueroa D, Austin CP, Metzker ML, Caskey CT, Wadelius C. Identification of the gene responsible for Best macular dystrophy. Nat Genet. 1998;19:241–7. - PubMed
-
- Burgess R, MacLaren RE, Davidson AE, Urquhart JE, Holder GE, Robson AG, Moore AT, Keefe RO, Black GC, Manson FD. ADVIRC is caused by distinct mutations in BEST1 that alter pre-mRNA splicing. J Med Genet. 2009;46:620–5. - PubMed
-
- Lafaut BA, Loeys B, Leroy BP, Spileers W, De LJJ, Kestelyn P. Clinical and electrophysiological findings in autosomal dominant vitreoretinochoroidopathy: report of a new pedigree. Graefes Arch Clin Exp Ophthalmol. 2001;239:575–82. - PubMed
-
- Reddy MA, Francis PJ, Berry V, Bradshaw K, Patel RJ, Maher ER, Kumar R, Bhattacharya SS, Moore AT. A clinical and molecular genetic study of a rare dominantly inherited syndrome (MRCS) comprising of microcornea, rod-cone dystrophy, cataract, and posterior staphyloma. Br J Ophthalmol. 2003;87:197–202. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
