Oskar-induced endocytic activation and actin remodeling for anchorage of the Drosophila germ plasm
- PMID: 21922042
- PMCID: PMC3173963
- DOI: 10.4161/bioa.1.3.17313
Oskar-induced endocytic activation and actin remodeling for anchorage of the Drosophila germ plasm
Abstract
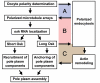
In many animals, germ-cell fate is specified by inheritance of the germ plasm, which is enriched in maternal RNAs and proteins. Assembly of the Drosophila germ (pole) plasm begins with the localization and translation of oskar (osk) RNA at the oocyte posterior pole. osk RNA produces two isoforms, long and short Osk. Short Osk recruits other pole plasm components, and long Osk restricts them to the oocyte cortex. Although molecular functions of long Osk remain mysterious, it is known to be involved in endocytic activation and actin cytoskeletal remodeling. We identified several vesicular trafficking machinery components that act downstream of long Osk in pole plasm assembly. These included the Rab5 effector protein Rabenosyn-5 (Rbsn-5) and the Golgi/endosomal protein Mon2, both of which were crucial for Osk-induced actin remodeling and the anchoring of pole plasm components. We propose that, in response to long Osk, the Rab5/Rbsn-5-dependent endocytic pathway promotes the formation of specialized vesicles, and Mon2 acts on these vesicles as a scaffold to instruct actin nucleators like Cappuccino and Spire to remodel the actin cytoskeleton, which anchors pole plasm components to the cortex. This mechanism may be applicable to the asymmetric localization of macromolecular structures such as protein-RNA complexes in other systems.
Figures


References
-
- Spradling AC. Development genetics of oogenesis. In: Bate M, Martinez-Arias A, editors. The Development of Drosophila melanogaster. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 1–70.
-
- Steinhauer J, Kalderon D. Microtubule polarity and axis formation in the Drosophila oocyte. Dev Dyn. 2006;235:1455–1468. - PubMed
-
- Clark I, Giniger E, Ruohola-Baker H, Jan LY, Jan YN. Transient posterior localization of a kinesin fusion protein reflects anteroposterior polarity of the Drosophila oocyte. Curr Biol. 1994;4:289–300. - PubMed
-
- Palacios IM, St. Johnston D. Kinesin light chain-independent function of the Kinesin heavy chain in cytoplasmic streaming and posterior localisation in the Drosophila oocyte. Development. 2002;129:5473–5485. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
