Endocannabinoid oxygenation by cyclooxygenases, lipoxygenases, and cytochromes P450: cross-talk between the eicosanoid and endocannabinoid signaling pathways
- PMID: 21923193
- PMCID: PMC3191732
- DOI: 10.1021/cr2002799
Endocannabinoid oxygenation by cyclooxygenases, lipoxygenases, and cytochromes P450: cross-talk between the eicosanoid and endocannabinoid signaling pathways
Figures
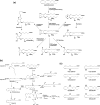
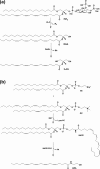


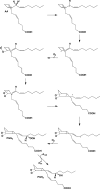
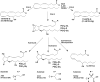

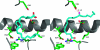

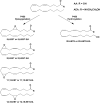
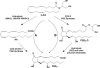
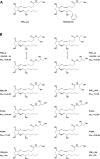

Similar articles
-
Oxidative metabolism of endocannabinoids.Prostaglandins Leukot Essent Fatty Acids. 2002 Feb-Mar;66(2-3):211-20. doi: 10.1054/plef.2001.0359. Prostaglandins Leukot Essent Fatty Acids. 2002. PMID: 12052037 Review.
-
Endocannabinoids and their oxygenation by cyclo-oxygenases, lipoxygenases and other oxygenases.Biochim Biophys Acta. 2015 Apr;1851(4):366-76. doi: 10.1016/j.bbalip.2014.12.015. Epub 2014 Dec 24. Biochim Biophys Acta. 2015. PMID: 25543004 Review.
-
Non-redundant functions of cyclooxygenases: oxygenation of endocannabinoids.J Biol Chem. 2008 Mar 28;283(13):8065-9. doi: 10.1074/jbc.R800005200. Epub 2008 Feb 4. J Biol Chem. 2008. PMID: 18250160 Free PMC article. Review.
-
Oxygenation of Anandamide by Lipoxygenases.Methods Mol Biol. 2023;2576:307-316. doi: 10.1007/978-1-0716-2728-0_26. Methods Mol Biol. 2023. PMID: 36152198
-
Eicosanoids: generation and detection in mammalian cells.Methods Mol Biol. 2009;462:5-23. Methods Mol Biol. 2009. PMID: 19160658 Review.
Cited by
-
Pharmacognosy and Effects of Cannabinoids in the Vascular System.ACS Pharmacol Transl Sci. 2022 Oct 28;5(11):1034-1049. doi: 10.1021/acsptsci.2c00141. eCollection 2022 Nov 11. ACS Pharmacol Transl Sci. 2022. PMID: 36407955 Free PMC article. Review.
-
Monoacylglycerol lipase inhibitors: modulators for lipid metabolism in cancer malignancy, neurological and metabolic disorders.Acta Pharm Sin B. 2020 Apr;10(4):582-602. doi: 10.1016/j.apsb.2019.10.006. Epub 2019 Oct 18. Acta Pharm Sin B. 2020. PMID: 32322464 Free PMC article. Review.
-
Fluorescent indomethacin-dansyl conjugates utilize the membrane-binding domain of cyclooxygenase-2 to block the opening to the active site.J Biol Chem. 2019 May 31;294(22):8690-8698. doi: 10.1074/jbc.RA119.007405. Epub 2019 Apr 18. J Biol Chem. 2019. PMID: 31000626 Free PMC article.
-
The Endocannabinoid System as a Therapeutic Target in Glaucoma.Neural Plast. 2016;2016:9364091. doi: 10.1155/2016/9364091. Epub 2016 Jan 12. Neural Plast. 2016. PMID: 26881140 Free PMC article. Review.
-
Plant-Derived and Endogenous Cannabinoids in Epilepsy.Clin Drug Investig. 2016 May;36(5):331-40. doi: 10.1007/s40261-016-0379-x. Clin Drug Investig. 2016. PMID: 26892745 Review.
References
-
- Devane W. A.; Dysarz F. A. 3rd; Johnson M. R.; Melvin L. S.; Howlett A. C. Mol. Pharmacol. 1988, 34, 605. - PubMed
-
- Matsuda L. A.; Lolait S. J.; Brownstein M. J.; Young A. C.; Bonner T. I. Nature 1990, 346, 561. - PubMed
-
- Munro S.; Thomas K. L.; Abu-Shaar M. Nature 1993, 365, 61. - PubMed
-
- Devane W. A.; Hanus L.; Breuer A.; Pertwee R. G.; Stevenson L. A.; Griffin G.; Gibson D.; Mandelbaum A.; Etinger A.; Mechoulam R. Science 1992, 258, 1946. - PubMed
-
- Sugiura T.; Kondo S.; Sukagawa A.; Nakane S.; Shinoda A.; Itoh K.; Yamashita A.; Waku K. Biochem. Biophys. Res. Commun. 1995, 215, 89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

