RbsB (NTHI_0632) mediates quorum signal uptake in nontypeable Haemophilus influenzae strain 86-028NP
- PMID: 21923771
- PMCID: PMC3609414
- DOI: 10.1111/j.1365-2958.2011.07831.x
RbsB (NTHI_0632) mediates quorum signal uptake in nontypeable Haemophilus influenzae strain 86-028NP
Abstract
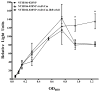
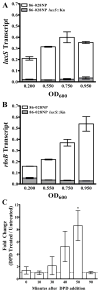
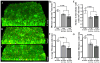
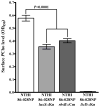
Nontypeable Haemophilus influenzae (NTHI) is a respiratory commensal and opportunistic pathogen, which persists within biofilms on airway mucosal surfaces. For many species, biofilm formation is impacted by quorum signalling. Our prior work shows that production of autoinducer-2 (AI-2) promotes biofilm development and persistence for NTHI 86-028NP. NTHI 86-028NP encodes an ABC transporter annotated as a ribose transport system that includes a protein (RbsB) with similarity to the Escherichia coli LsrB and Aggregatibacter actinomycetemcomitans RbsB proteins that bind AI-2. In this study, inactivation of rbsB significantly reduced uptake of AI-2 and the AI-2 precursor dihydroxypentanedione (DPD) by NTHI 86-028NP. Moreover, DPD uptake was not competitively inhibited by ribose or other pentose sugars. Transcript levels of rbsB increased in response to DPD and as bacteria approached stationary-phase growth. The NTHI 86-028NP rbsB mutant also formed biofilms with significantly reduced thickness and total biomass and reduced surface phosphorylcholine, similar to a luxS mutant. Infection studies revealed that loss of rbsB impaired bacterial persistence in the chinchilla middle ear, similar to our previous results with luxS mutants. Based on these data, we conclude that in NTHI 86-028NP, RbsB is a LuxS/AI-2 regulated protein that is required for uptake of and response to AI-2.
© 2011 Blackwell Publishing Ltd.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

