LIM kinase 1 - dependent cofilin 1 pathway and actin dynamics mediate nuclear retinoid receptor function in T lymphocytes
- PMID: 21923909
- PMCID: PMC3187726
- DOI: 10.1186/1471-2199-12-41
LIM kinase 1 - dependent cofilin 1 pathway and actin dynamics mediate nuclear retinoid receptor function in T lymphocytes
Erratum in
- BMC Mol Biol. 2012;13:14. Aguilera-Gutierrez, Angelica [added]
Abstract
Background: It is known that retinoid receptor function is attenuated during T cell activation, a phenomenon that involves actin remodeling, suggesting that actin modification may play a role in such inhibition. Here we have investigated the role of actin dynamics and the effect of actin cytoskeleton modifying agents on retinoid receptor-mediated transactivation.
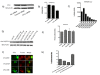
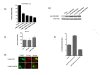
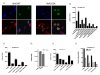
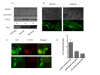
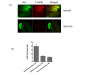
Results: Agents that disturb the F-actin assembly or disassembly attenuated receptor-mediated transcription indicating that actin cytoskeletal homeostasis is important for retinoid receptor function. Overexpression or siRNA-induced knockdown of cofilin-1 (CFL1), a key regulator of F-actin assembly, induced the loss of receptor function. In addition, expression of either constitutively active or inactive/dominant-negative mutants of CFL1or CFL1 kinase LIMK1 induced loss of receptor function suggesting a critical role of the LIMK1-mediated CFL1 pathway in receptor-dependent transcription. Further evidence of the role of LMK1/CFL1-mediated actin dynamics, was provided by studying the effect of Nef, an actin modifying HIV-1 protein, on receptor function. Expression of Nef induced phosphorylation of CFL1 at serine 3 and LIMK1 at threonine 508, inhibited retinoid-receptor mediated reporter activity, and the expression of a number of genes that contain retinoid receptor binding sites in their promoters. The results suggest that the Nef-mediated inhibition of receptor function encompasses deregulation of actin filament dynamics by LIMK1 activation and phosphorylation of CFL1.
Conclusion: We have identified a critical role of LIMK1-mediated CFL1 pathway and actin dynamics in modulating retinoid receptor mediated function and shown that LIMK1-mediated phosphocycling of CFL1 plays a crucial role in maintaining actin homeostasis and receptor activity. We suggest that T cell activation-induced repression of nuclear receptor-dependent transactivation is in part through the modification of actin dynamics.
Figures
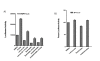
References
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

