Tracking human multiple myeloma xenografts in NOD-Rag-1/IL-2 receptor gamma chain-null mice with the novel biomarker AKAP-4
- PMID: 21923911
- PMCID: PMC3189930
- DOI: 10.1186/1471-2407-11-394
Tracking human multiple myeloma xenografts in NOD-Rag-1/IL-2 receptor gamma chain-null mice with the novel biomarker AKAP-4
Abstract
Background: Multiple myeloma (MM) is a fatal malignancy ranking second in prevalence among hematological tumors. Continuous efforts are being made to develop innovative and more effective treatments. The preclinical evaluation of new therapies relies on the use of murine models of the disease.
Methods: Here we describe a new MM animal model in NOD-Rag1null IL2rgnull (NRG) mice that supports the engraftment of cell lines and primary MM cells that can be tracked with the tumor antigen, AKAP-4.
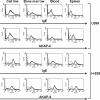
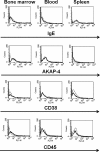
Results: Human MM cell lines, U266 and H929, and primary MM cells were successfully engrafted in NRG mice after intravenous administration, and were found in the bone marrow, blood and spleen of tumor-challenged animals. The AKAP-4 expression pattern was similar to that of known MM markers, such as paraproteins, CD38 and CD45.
Conclusions: We developed for the first time a murine model allowing for the growth of both MM cell lines and primary cells in multifocal sites, thus mimicking the disease seen in patients. Additionally, we validated the use of AKAP-4 antigen to track tumor growth in vivo and to specifically identify MM cells in mouse tissues. We expect that our model will significantly improve the pre-clinical evaluation of new anti-myeloma therapies.
Figures


Similar articles
-
Intratibial injection of human multiple myeloma cells in NOD/SCID IL-2Rγ(null) mice mimics human myeloma and serves as a valuable tool for the development of anticancer strategies.PLoS One. 2013 Nov 6;8(11):e79939. doi: 10.1371/journal.pone.0079939. eCollection 2013. PLoS One. 2013. PMID: 24223204 Free PMC article.
-
Mouse models of mantle cell lymphoma, complex changes in gene expression and phenotype of engrafted MCL cells: implications for preclinical research.Lab Invest. 2014 Jul;94(7):806-17. doi: 10.1038/labinvest.2014.61. Epub 2014 May 26. Lab Invest. 2014. PMID: 24862967
-
NOD-scidIl2rg (tm1Wjl) and NOD-Rag1 (null) Il2rg (tm1Wjl) : a model for stromal cell-tumor cell interaction for human colon cancer.Dig Dis Sci. 2014 Jun;59(6):1169-79. doi: 10.1007/s10620-014-3168-5. Epub 2014 May 6. Dig Dis Sci. 2014. PMID: 24798995 Free PMC article.
-
Non-obese diabetic-recombination activating gene-1 (NOD-Rag1 null) interleukin (IL)-2 receptor common gamma chain (IL2r gamma null) null mice: a radioresistant model for human lymphohaematopoietic engraftment.Clin Exp Immunol. 2008 Nov;154(2):270-84. doi: 10.1111/j.1365-2249.2008.03753.x. Epub 2008 Sep 8. Clin Exp Immunol. 2008. PMID: 18785974 Free PMC article.
-
Long term human reconstitution and immune aging in NOD-Rag (-)-γ chain (-) mice.Immunobiology. 2014 Feb;219(2):131-7. doi: 10.1016/j.imbio.2013.08.013. Epub 2013 Sep 5. Immunobiology. 2014. PMID: 24094417 Free PMC article.
Cited by
-
Patient-derived tumor models are attractive tools to repurpose drugs for ovarian cancer treatment: pre-clinical updates.Oncotarget. 2022 Mar 24;13:553-575. doi: 10.18632/oncotarget.28220. eCollection 2022. Oncotarget. 2022. PMID: 35359749 Free PMC article. Review.
-
Myeloma Cell Dynamics in Response to Treatment Supports a Model of Hierarchical Differentiation and Clonal Evolution.Clin Cancer Res. 2016 Aug 15;22(16):4206-4214. doi: 10.1158/1078-0432.CCR-15-2793. Epub 2016 Mar 22. Clin Cancer Res. 2016. PMID: 27006493 Free PMC article.
-
AKAP4 mediated tumor malignancy in esophageal cancer.Am J Transl Res. 2016 Feb 15;8(2):597-605. eCollection 2016. Am J Transl Res. 2016. PMID: 27158351 Free PMC article.
-
Human cancer growth and therapy in immunodeficient mouse models.Cold Spring Harb Protoc. 2014 Jul 1;2014(7):694-708. doi: 10.1101/pdb.top073585. Cold Spring Harb Protoc. 2014. PMID: 24987146 Free PMC article. Review.
-
Identification of CD8+ T-cell epitope from multiple myeloma-specific antigen AKAP4.Front Immunol. 2022 Jul 28;13:927804. doi: 10.3389/fimmu.2022.927804. eCollection 2022. Front Immunol. 2022. PMID: 35967402 Free PMC article.
References
-
- Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Cronin K, Chen HS, Feuer EJ, Stinchcomb DG, Edwards BK, eds. SEER Cancer Statistics Review, 1975-2007, National Cancer Institute. Bethesda MD;
-
- Kastritis E, Zervas K, Symeonidis A, Terpos E, Delimbassi S, Anagnostopoulos N, Michali E, Zomas A, Katodritou E, Gika D, Pouli A, Christoulas D, Roussou M, Kartasis Z, Economopoulos T, Dimopoulos MA. Improved survival of patients with multiple myeloma after the introduction of novel agents and the applicability of the International Staging System (ISS): an analysis of the Greek Myeloma Study Group (GMSG) Leukemia. 2009;23(6):1152–1157. doi: 10.1038/leu.2008.402. - DOI - PubMed
-
- Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Zeldenrust SR, Dingli D, Russell SJ, Lust JA, Greipp PR, Kyle RA, Gertz MA. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111(5):2516–2520. doi: 10.1182/blood-2007-10-116129. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

