Effects of metastasis-associated in colon cancer 1 inhibition by small hairpin RNA on ovarian carcinoma OVCAR-3 cells
- PMID: 21923915
- PMCID: PMC3182136
- DOI: 10.1186/1756-9966-30-83
Effects of metastasis-associated in colon cancer 1 inhibition by small hairpin RNA on ovarian carcinoma OVCAR-3 cells
Abstract
Background: Metastasis-associated in colon cancer 1 (MACC1) is demonstrated to be up-regulated in several types of cancer, and can serve as biomarker for cancer invasion and metastasis. To investigate the relations between MACC1 and biological processes of ovarian cancer, MACC1 specific small hairpin RNA (shRNA) expression plasmids were used to investigate the effects of MACC1 inhibition on ovarian carcinoma OVCAR-3 cells.
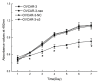
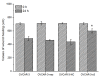
Methods: Expressions of MACC1 were detected in different ovarian tissues by immunohistochemistry. MACC1 specific shRNA expression plasmids were constructed and transfected into OVCAR-3 cells. Then, expressions of MACC1 were examined by reverse transcription polymerase chain reaction (RT-PCR) and Western blot. Cell proliferation was observed by MTT and monoplast colony formation assay. Flow cytometry and TUNEL assay were used to measure cell apoptosis. Cell migration was assessed by wound healing and transwell migration assay. Matrigel invasion and xenograft model assay were performed to analyze the potential of cell invasion. Activities of Met, MEK1/2, ERK1/2, Akt, cyclinD1, caspase3 and MMP2 protein were measured by Western blot.
Results: Overexpressions of MACC1 were detected in ovarian cancer tissues. Expression of MACC1 in OVCAR-3 cells was significantly down-regulated by MACC1 specific small hairpin RNA. In OVCAR-3 cells, down-regulation of MACC1 resulted in significant inhibition of cell proliferation, migration and invasion, meanwhile obvious enhancement of apoptosis. As a consequence of MACC1 knockdown, expressions of Met, p-MEK1/2, p-ERK1/2, cyclinD1 and MMP2 protein decreased, level of cleaved capase3 was increased.
Conclusions: RNA interference (RNAi) against MACC1 could serve as a promising intervention strategy for gene therapy of ovarian carcinoma, and the antitumor effects of MACC1 knockdown might involve in the inhibition of HGF/Met and MEK/ERK pathways.
Figures



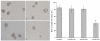



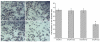




Similar articles
-
MicroRNA-338-3p suppresses ovarian cancer cells growth and metastasis: implication of Wnt/catenin beta and MEK/ERK signaling pathways.J Exp Clin Cancer Res. 2019 Dec 16;38(1):494. doi: 10.1186/s13046-019-1494-3. J Exp Clin Cancer Res. 2019. Retraction in: J Exp Clin Cancer Res. 2024 Feb 15;43(1):48. doi: 10.1186/s13046-024-02978-0. PMID: 31842953 Free PMC article. Retracted.
-
Targeting MACC1 by RNA interference inhibits proliferation and invasion of bladder urothelial carcinoma in T24 cells.Int J Clin Exp Pathol. 2015 Jul 1;8(7):7937-44. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26339359 Free PMC article.
-
Wilms tumor gene protein 1 is associated with ovarian cancer metastasis and modulates cell invasion.Cancer. 2008 Apr 1;112(7):1632-41. doi: 10.1002/cncr.23341. Cancer. 2008. PMID: 18260155
-
MACC1 revisited - an in-depth review of a master of metastasis.Biomark Res. 2024 Nov 23;12(1):146. doi: 10.1186/s40364-024-00689-4. Biomark Res. 2024. PMID: 39580452 Free PMC article. Review.
-
MACC1-induced migration in tumors: Current state and perspective.Front Oncol. 2023 Mar 27;13:1165676. doi: 10.3389/fonc.2023.1165676. eCollection 2023. Front Oncol. 2023. PMID: 37051546 Free PMC article. Review.
Cited by
-
Metastasis-associated in colon cancer-1 in gastric cancer: Beyond metastasis.World J Gastroenterol. 2016 Aug 7;22(29):6629-37. doi: 10.3748/wjg.v22.i29.6629. World J Gastroenterol. 2016. PMID: 27547006 Free PMC article. Review.
-
Circulating MACC1 transcripts in colorectal cancer patient plasma predict metastasis and prognosis.PLoS One. 2012;7(11):e49249. doi: 10.1371/journal.pone.0049249. Epub 2012 Nov 14. PLoS One. 2012. PMID: 23166620 Free PMC article.
-
Prognostic value and clinical pathology of MACC-1 and c-MET expression in gastric carcinoma.Pathol Oncol Res. 2013 Oct;19(4):821-32. doi: 10.1007/s12253-013-9650-0. Epub 2013 Jul 1. Pathol Oncol Res. 2013. PMID: 23812675
-
Analysis of HGF, MACC1, C-met and apoptosis-related genes in cervical carcinoma mice.Mol Biol Rep. 2014 Mar;41(3):1247-56. doi: 10.1007/s11033-013-2969-5. Epub 2014 Jan 28. Mol Biol Rep. 2014. PMID: 24469707
-
PIM Kinases and Their Relevance to the PI3K/AKT/mTOR Pathway in the Regulation of Ovarian Cancer.Biomolecules. 2018 Feb 4;8(1):7. doi: 10.3390/biom8010007. Biomolecules. 2018. PMID: 29401696 Free PMC article. Review.
References
-
- Shirahata A, Shinmura K, Kitamura Y, Sakuraba K, Yokomizo K, Goto T, Mizukami H, Saito M, Ishibashi K, Kigawa G, Nemoto H, Hibi K. MACC1 as a marker for advanced colorectal carcinoma. Anticancer Res. 2010;30:2689–2692. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

