A longitudinal study of stavudine-associated toxicities in a large cohort of South African HIV infected subjects
- PMID: 21923929
- PMCID: PMC3189398
- DOI: 10.1186/1471-2334-11-244
A longitudinal study of stavudine-associated toxicities in a large cohort of South African HIV infected subjects
Abstract
Background: There has been major improvement in the survival of HIV-1 infected individuals since the South African Government introduced highly active anti-retroviral therapy (HAART) in the public sector in 2004. This has brought new challenges which include the effects of stavudine-related toxicities.
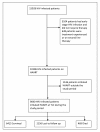
Methods: Prospective analysis of a cohort of 9040 HIV-infected adults who were initiated on HAART at the Themba Lethu Clinic (TLC) in Johannesburg between April 1, 2004 to December 31, 2007, and followed up until June 30, 2008.
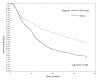
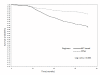
Results: Amongst the 9040 study subjects, 8497(94%) were on stavudine based therapy and 5962 (66%) were women. The median baseline CD4 count was 81 cells/mm3 (IQR 29-149). Median follow up on HAART was 19 months (IQR: 9.1-31.6). The proportion of HAART-related side effects for stavudine compared to non-stavudine containing regimens were, respectively: peripheral neuropathy,17.1% vs. 11.2% (p < 0.001); symptomatic hyperlactataemia, 5.7% vs. 2.2% (p < 0.0005); lactic acidosis, 2.5 vs. 1.3% (p = 0.072); lipoatrophy, 7.3% vs. 4.6% (p < 0.05). Among those on stavudine-based regimens, incidence rates for peripheral neuropathy were 12.1 cases/100 person-years (95%CI 7.0-19.5), symptomatic hyperlactataemia 3.6 cases/100 person-years (95%CI 1.2-7.5), lactic acidosis 1.6 cases/100 person-years (95%CI 0.4-5.2) and lipoatrophy 4.6 cases/100 person-years (95%CI 2.1-9.6). Females experienced more toxicity when compared to males in terms of symptomatic hyperlactataemia (p < 0.0001), lactic acidosis (p < 0.0001), lipoatrophy (p < 0.0001) and hypertension (p < 0.05).
Conclusions: We demonstrate significant morbidity associated with stavudine. These data support the latest WHO guidelines, and provide additional evidence for other resource limited HAART rollout programs considering the implementation of non-stavudine based regimens as first line therapy.
Figures


References
-
- UNAIDS AIDS epidemic update. 2009. http://data.unaids.org/pub/Report/2009/JC1700_Epi_Update_2009_en.pdf Last accessed on the 07/12//2010.
-
- Department of Health. National Antenatal Sentinel HIV and Syphilis Prevalence Survey in South Africa, 2009. 2010. http://www.health-e.org.za/documents/85d3dad6136e8ca9d02cceb7f4a36145.pdf Last accessed on the 31/08//2011.
-
- Palella FJ Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. - DOI - PubMed
-
- Hogg RS, O'Shaughnessy MV, Gataric N, Yip B, Craib K, Schechter MT, Montaner JS. Decline in deaths from AIDS due to new antiretrovirals. Lancet. 1997;349:1294. - PubMed
-
- Wit FW, van Leeuwen R, Weverling GJ, Jurriaans S, Nauta K, Steingrover R, Schuijtemaker J, Eyssen X, Fortuin D, Weeda M, de Wolf F, Reiss P, Danner SA, Lange JM. Outcome and predictors of failure of highly active antiretroviral therapy: one-year follow-up of a cohort of human immunodeficiency virus type 1-infected persons. J Infect Dis. 1999;179:790–798. doi: 10.1086/314675. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

