The itty-bitty time machine genetics of the cyanobacterial circadian clock
- PMID: 21924974
- PMCID: PMC3319097
- DOI: 10.1016/B978-0-12-387690-4.00002-7
The itty-bitty time machine genetics of the cyanobacterial circadian clock
Abstract
The cyanobacterium Synechococcus elongatus PCC 7942 has been used as the prokaryotic model system for the study of circadian rhythms for the past two decades. Its genetic malleability has been instrumental in the discovery of key input, oscillator, and output components and has also provided monumental insights into the mechanism by which proteins function to maintain and dictate 24-h time. In addition, basic research into the prokaryotic system has led to interesting advances in eukaryotic circadian mechanisms. Undoubtedly, continued genetic and mutational analyses of this single-celled cyanobacterium will aid in teasing out the intricacies of the Kai-based circadian clock to advance our understanding of this system as well as other more "complex" systems.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
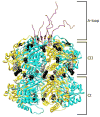
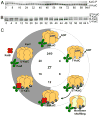


References
-
- Arita K, Hashimoto H, Igari K, Akaboshi M, Kutsuna S, Sato M, Shimizu T. Structural and biochemical characterization of a cyanobacterium circadian clock-modifier protein. J Biol Chem. 2007;282:1128–35. - PubMed
-
- Aschoff J. Freerunning and entrained circadian rhythms. Handbook of behavioral neurobiology: Biological rhythms. 1981:81–93.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

