Calcium channel blocking as a therapeutic strategy for Alzheimer's disease: the case for isradipine
- PMID: 21925266
- PMCID: PMC3275089
- DOI: 10.1016/j.bbadis.2011.08.013
Calcium channel blocking as a therapeutic strategy for Alzheimer's disease: the case for isradipine
Abstract
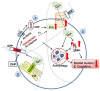
Alzheimer's disease is the most devastating neurodegenerative disorder in the elderly, yet treatment options are severely limited. The drug development effort to modify Alzheimer's disease pathology by intervention at beta amyloid production sites has been largely ineffective or inconclusive. The greatest challenge has been to identify and define downstream mechanisms reliably predictive of clinical symptoms. Beta amyloid accumulation leads to dysregulation of intracellular calcium by plasma membrane L-type calcium channels located on neuronal somatodendrites and axons in the hippocampus and cortex. Paradoxically, L-type calcium channel subtype Ca(v)1.2 also promotes synaptic plasticity and spatial memory. Increased intracellular calcium modulates amyloid precursor protein processing and affects multiple downstream pathways including increased hyperphosphorylated tau and suppression of autophagy. Isradipine is a Federal Drug Administration-approved dihydropyridine calcium channel blocker that binds selectively to Ca(v)1.2 in the hippocampus. Our studies have shown that isradipine in vitro attenuates beta amyloid oligomer toxicity by suppressing calcium influx into cytoplasm and by suppressing Ca(v)1.2 expression. We have previously shown that administration of isradipine to triple transgenic animal model for Alzheimer's disease was well-tolerated. Our results further suggest that isradipine became bioavailable, lowered tau burden, and improved autophagy function in the brain. A better understanding of brain pharmacokinetics of calcium channel blockers will be critical for designing new experiments with appropriate drug doses in any future clinical trials for Alzheimer's disease. This review highlights the importance of Ca(v)1.2 channel overexpression, the accumulation of hyperphosphorylated tau and suppression of autophagy in Alzheimer's disease and modulation of this pathway by isradipine.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures
References
-
- White JA, McKinney BC, John MC, Powers PA, Kamp TJ, Murphy GG. Conditional forebrain deletion of the L-type calcium channel Ca V 1.2 disrupts remote spatial memories in mice. Learn Mem. 2008;15:1–5. - PubMed
-
- Tippens AL, Pare JF, Langwieser N, Moosmang S, Milner TA, Smith Y, Lee A. Ultrastructural evidence for pre- and postsynaptic localization of Cav1.2 L-type Ca2+ channels in the rat hippocampus. J Comp Neurol. 2008;506:569–583. - PubMed
-
- Lacinova L, Moosmang S, Langwieser N, Hofmann F, Kleppisch T. Cav1.2 calcium channels modulate the spiking pattern of hippocampal pyramidal cells. Life Sci. 2008;82:41–49. - PubMed
-
- Moosmang S, Haider N, Klugbauer N, Adelsberger H, Langwieser N, Muller J, Stiess M, Marais E, Schulla V, Lacinova L, Goebbels S, Nave KA, Storm DR, Hofmann F, Kleppisch T. Role of hippocampal Cav1.2 Ca2+ channels in NMDA receptor-independent synaptic plasticity and spatial memory. J Neurosci. 2005;25:9883–9892. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

