Gut-tropic T cells that express integrin α4β7 and CCR9 are required for induction of oral immune tolerance in mice
- PMID: 21925467
- PMCID: PMC3222333
- DOI: 10.1053/j.gastro.2011.09.015
Gut-tropic T cells that express integrin α4β7 and CCR9 are required for induction of oral immune tolerance in mice
Abstract
Background & aims: Induction of oral immune tolerance (OT) blocks proinflammatory responses to orally administered antigens and might be used to treat autoimmune conditions. We investigated whether gut-tropic T cells that express the integrin α4β7 and the chemokine receptor CCR9 are required for OT.
Methods: Skin delayed-type hypersensitivity and experimental autoimmune encephalomyelitis were used to monitor OT in mice. To assess the role of receptors that mediate localization of lymphocytes to the gut (gut-homing receptors) in induction of OT, we studied CCR9(-/-) and β7(-/-) mice and also blocked the α4β7 ligand MAdCAM-1 in wild-type mice. We used DEREG and Scurfy mice to assess the role of Foxp3(+) regulatory T cells (Treg) and IL-10(-/-) and IL-10Rβ(-/-) mice to examine the role of interleukin (IL)-10 in induction of OT.
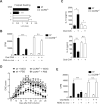
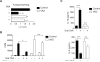
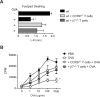
Results: OT could not be induced in CCR9(-/-) or β7(-/-) mice, or when MAdCAM-1 was blocked in wild-type mice, indicating that gut-homing receptors are required for oral tolerization. Consistent with the role of all-trans retinoic acid in inducing gut-homing T cells, OT could not be induced in mice depleted of vitamin A. OT was rescued in CCR9(-/-) mice following adoptive transfer of wild-type T cells, but not CCR9(-/-) or β7(-/-) T cells. Gut-homing T cells are therefore necessary and sufficient to induce OT. Wild-type Treg and IL-10 were required to restore OT to CCR9(-/-) mice, indicating that homing and functional differentiation of IL-10-producing Treg in the gut is required for OT. Conversely, transfer of CCR9(-/-) or β7(-/-) T cells to wild-type mice partially inhibited OT.
Conclusions: Expression of CCR9 and α4β7 on T cells and their subsequent localization to the gut is required for induction of OT in mice. Therapies designed to block gut-homing receptors might, under some conditions, interfere with normal tolerogenic mechanisms in the intestinal mucosa.
Copyright © 2011 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Chen Y, Inobe J, Marks R, Gonnella P, Kuchroo VK, Weiner HL. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature. 1995;376:177–80. - PubMed
-
- Dubois B, Joubert G, Gomez de Aguero M, Gouanvic M, Goubier A, Kaiserlian D. Sequential role of plasmacytoid dendritic cells and regulatory T cells in oral tolerance. Gastroenterology. 2009;137:1019–28. - PubMed
-
- Zhang X, Izikson L, Liu L, Weiner HL. Activation of CD25(+)CD4(+) regulatory T cells by oral antigen administration. J Immunol. 2001;167:4245–53. - PubMed
-
- Spahn TW, Weiner HL, Rennert PD, Lugering N, Fontana A, Domschke W, Kucharzik T. Mesenteric lymph nodes are critical for the induction of high-dose oral tolerance in the absence of Peyer's patches. Eur J Immunol. 2002;32:1109–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

