Human immunodeficiency virus infects human seminal vesicles in vitro and in vivo
- PMID: 21925468
- PMCID: PMC3204009
- DOI: 10.1016/j.ajpath.2011.08.005
Human immunodeficiency virus infects human seminal vesicles in vitro and in vivo
Abstract
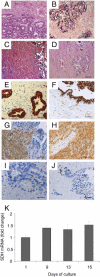
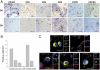
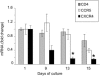
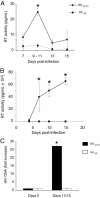
Semen represents the main vector of HIV dissemination worldwide, yet the origin of HIV in semen remains unclear. Viral populations distinct from those found in blood have been observed in semen, indicating local viral replication within the male genital tract. The seminal vesicles, the secretions of which constitute more than 60% of the seminal fluid, could represent a major source of virus in semen. This study is the first to investigate the susceptibility of human seminal vesicles to HIV infection both in vitro and in vivo. We developed and characterized an organotypic culture of human seminal vesicles to test for target cells and HIV infection, and, in parallel, analyzed the seminal vesicle tissues from HIV-infected donors. In vitro, in contrast to HIV-1 X4, HIV-1 R5 exposure induced productive infection. Infected cells consisted primarily of resident CD163(+) macrophages, often located close to the lumen. In vivo, HIV protein and RNA were also detected primarily in seminal vesicle macrophages in seven of nine HIV-infected donors, some of whom were receiving prolonged suppressive highly active antiretroviral therapy. These results demonstrate that human seminal vesicles support HIV infection in vitro and in vivo and, therefore, have the potential to contribute virus to semen. The presence of infected cells in the seminal vesicles of treated men with undetectable viremia suggests that this organ could constitute a reservoir for HIV.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Detection of Simian Immunodeficiency Virus in Semen, Urethra, and Male Reproductive Organs during Efficient Highly Active Antiretroviral Therapy.J Virol. 2015 Jun;89(11):5772-87. doi: 10.1128/JVI.03628-14. Epub 2015 Apr 1. J Virol. 2015. PMID: 25833047 Free PMC article.
-
Human immunodeficiency virus type 1 in the semen of men receiving highly active antiretroviral therapy.N Engl J Med. 1998 Dec 17;339(25):1803-9. doi: 10.1056/NEJM199812173392502. N Engl J Med. 1998. PMID: 9854115
-
Seminal Simian Immunodeficiency Virus in Chronically Infected Cynomolgus Macaques Is Dominated by Virus Originating from Multiple Genital Organs.J Virol. 2018 Jun 29;92(14):e00133-18. doi: 10.1128/JVI.00133-18. Print 2018 Jul 15. J Virol. 2018. PMID: 29720516 Free PMC article.
-
[The male genital tract: A host for HIV].Gynecol Obstet Fertil. 2007 Dec;35(12):1245-50. doi: 10.1016/j.gyobfe.2007.09.017. Epub 2007 Nov 26. Gynecol Obstet Fertil. 2007. PMID: 18035579 Review. French.
-
HIV infection of the male genital tract--consequences for sexual transmission and reproduction.Int J Androl. 2010 Feb;33(1):e98-108. doi: 10.1111/j.1365-2605.2009.00973.x. Epub 2009 Jun 15. Int J Androl. 2010. PMID: 19531082 Free PMC article. Review.
Cited by
-
Detection of Simian Immunodeficiency Virus in Semen, Urethra, and Male Reproductive Organs during Efficient Highly Active Antiretroviral Therapy.J Virol. 2015 Jun;89(11):5772-87. doi: 10.1128/JVI.03628-14. Epub 2015 Apr 1. J Virol. 2015. PMID: 25833047 Free PMC article.
-
The HIV Reservoir in Monocytes and Macrophages.Front Immunol. 2019 Jun 26;10:1435. doi: 10.3389/fimmu.2019.01435. eCollection 2019. Front Immunol. 2019. PMID: 31297114 Free PMC article. Review.
-
HIV persistence in the setting of antiretroviral therapy: when, where and how does HIV hide?J Virus Erad. 2015 Apr;1(2):59-66. doi: 10.1016/S2055-6640(20)30490-8. J Virus Erad. 2015. PMID: 26448966 Free PMC article.
-
Viral infections and implications for male reproductive health.Asian J Androl. 2021 Jul-Aug;23(4):335-347. doi: 10.4103/aja.aja_82_20. Asian J Androl. 2021. PMID: 33473014 Free PMC article.
-
A single cell atlas of the mouse seminal vesicle.G3 (Bethesda). 2025 May 8;15(5):jkaf045. doi: 10.1093/g3journal/jkaf045. G3 (Bethesda). 2025. PMID: 40036847 Free PMC article.
References
-
- Royce R.A., Sena A., Cates W., Jr, Cohen M.S. Sexual transmission of HIV. N Engl J Med. 1997;336:1072–1078. - PubMed
-
- Ghosn J., Viard J.P., Katlama C., de Almeida M., Tubiana R., Letourneur F., Aaron L., Goujard C., Salmon D., Leruez-Ville M., Rouzioux C., Chaix M.L. Evidence of genotypic resistance diversity of archived and circulating viral strains in blood and semen of pre-treated HIV-infected men. AIDS. 2004;18:447–457. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

