Role of FAK in S1P-regulated endothelial permeability
- PMID: 21925517
- PMCID: PMC3684237
- DOI: 10.1016/j.mvr.2011.08.012
Role of FAK in S1P-regulated endothelial permeability
Abstract
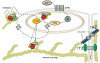
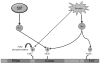
The vascular endothelium serves as a semi-selective barrier between the circulating contents of the blood and the tissues through which they flow. Disruption of this barrier results in significant organ dysfunction during devastating inflammatory syndromes such as sepsis and acute lung injury (ALI). Sphingosine 1-phosphate (S1P) is an endogenous lipid regulator of endothelial permeability that produces potent barrier enhancement via actin and junctional protein rearrangement and resultant cytoskeletal changes. A key effector protein in this S1P response is focal adhesion kinase (FAK), a highly conserved cytoplasmic tyrosine kinase involved in the engagement of integrins and assembly of focal adhesions (FA) through the catalysis of multiple downstream signals. After stimulation by S1P, endothelial FAK undergoes specific tyrosine phosphorylation that results in activation of the kinase and dynamic interactions with other effector molecules to improve the endothelial barrier. FAK participates in peripheral actin cytoskeletal rearrangement as well as cell-matrix (FA) and cell-cell (adherens junction) junctional complex strengthening that combine to decrease vascular permeability. This review summarizes the current knowledge of the role of FAK in mediating enhanced endothelial barrier function by S1P.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


References
-
- Avizienyte E, Wyke AW, et al. Src-induced de-regulation of E-cadherin in colon cancer cells requires integrin signalling. Nat Cell Biol. 2002;4(8):632–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

