Xpa deficiency reduces CAG trinucleotide repeat instability in neuronal tissues in a mouse model of SCA1
- PMID: 21926083
- PMCID: PMC3221534
- DOI: 10.1093/hmg/ddr421
Xpa deficiency reduces CAG trinucleotide repeat instability in neuronal tissues in a mouse model of SCA1
Abstract
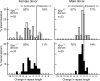
Expansion of trinucleotide repeats (TNRs) is responsible for a number of human neurodegenerative disorders. The molecular mechanisms that underlie TNR instability in humans are not clear. Based on results from model systems, several mechanisms for instability have been proposed, all of which focus on the ability of TNRs to form alternative structures during normal DNA transactions, including replication, DNA repair and transcription. These abnormal structures are thought to trigger changes in TNR length. We have previously shown that transcription-induced TNR instability in cultured human cells depends on several genes known to be involved in transcription-coupled nucleotide excision repair (NER). We hypothesized that NER normally functions to destabilize expanded TNRs. To test this hypothesis, we bred an Xpa null allele, which eliminates NER, into the TNR mouse model for spinocerebellar ataxia type 1 (SCA1), which carries an expanded CAG repeat tract at the endogenous mouse Sca1 locus. We find that Xpa deficiency does not substantially affect TNR instability in either the male or female germline; however, it dramatically reduces CAG repeat instability in neuronal tissues-striatum, hippocampus and cerebral cortex-but does not alter CAG instability in kidney or liver. The tissue-specific effect of Xpa deficiency represents a novel finding; it suggests that tissue-to-tissue variation in CAG repeat instability arises, in part, by different underlying mechanisms. These results validate our original findings in cultured human cells and suggest that transcription may induce NER-dependent TNR instability in neuronal tissues in humans.
Figures



References
-
- Orr H.T., Zoghbi H.Y. Trinucleotide repeat disorders. Annu. Rev. Neurosci. 2007;30:575–621. - PubMed
-
- Gatchel J.R., Zoghbi H.Y. Diseases of unstable repeat expansion: mechanisms and common principles. Nat. Rev. Genet. 2005;6:743–755. - PubMed
-
- Pearson C.E., Edamura K.N., Cleary J.D. Repeat instability: mechanisms of dynamic mutations. Nat. Rev. Genet. 2005;6:729–742. - PubMed
-
- Shelbourne P.F., Keller-McGandy C., Bi W.L., Yoon S.R., Dubeau L., Veitch N.J., Vonsattel J.P., Wexler N.S., Arnheim N., Augood S.J. Triplet repeat mutation length gains correlate with cell-type specific vulnerability in Huntington disease brain. Hum. Mol. Genet. 2007;16:1133–1142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

