Loss of memory B cells during chronic HIV infection is driven by Foxo3a- and TRAIL-mediated apoptosis
- PMID: 21926463
- PMCID: PMC3195482
- DOI: 10.1172/JCI59211
Loss of memory B cells during chronic HIV infection is driven by Foxo3a- and TRAIL-mediated apoptosis
Erratum in
- J Clin Invest. 2012 Jul 2;122(7):2704
Abstract
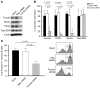
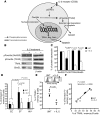
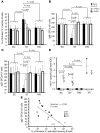
Loss of memory B cells occurs from the onset of HIV-1 infection and persists into the chronic stages of infection. Lack of survival of these cells, even in subjects being treated, could primarily be the consequence of an altered local microenvironment induced by HIV infection. In this study we showed that memory B cell survival was significantly decreased in aviremic successfully treated (ST) subjects compared with subjects who control viral load as a result of natural immunity (elite controller [EC]) or with uninfected control (HIV-) subjects. The lower survival levels observed in memory B cells from ST subjects were the result of disrupted IL-2 signaling that led to increased transcriptional activity of Foxo3a and increased expression of its proapoptotic target TRAIL. Notably, memory B cell survival in ST subjects was significantly enhanced by the addition of exogenous IL-2 in a Foxo3a-dependent manner. We further showed that Foxo3a silencing by siRNA resulted in decreased expression of TRAIL and apoptosis levels in memory B cells from ST subjects. Our results thus establish a direct role for Foxo3a/TRAIL signaling in the persistence of memory B cells and provide a mechanism for the reduced survival of memory B cells during HIV infection. This knowledge could be exploited for the development of therapeutic and preventative HIV vaccines.
Figures
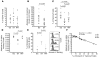


References
-
- Amadori A, et al. B cell activation during HIV-1 infection. II. Cell-to-cell interactions and cytokine requirement. J Immunol. 1991;146(1):57–62. - PubMed
-
- Samuelsson A, Brostrom C, van Dijk N, Sonnerborg A, Chiodi F. Apoptosis of CD4+ and CD19+ cells during human immunodeficiency virus type 1 infection--correlation with clinical progression, viral load, and loss of humoral immunity. Virology. 1997;238(2):180–188. doi: 10.1006/viro.1997.8790. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

