A clinical assessment of the Mucus Shaver: a device to keep the endotracheal tube free from secretions
- PMID: 21926595
- PMCID: PMC3405906
- DOI: 10.1097/CCM.0b013e31822e9fe3
A clinical assessment of the Mucus Shaver: a device to keep the endotracheal tube free from secretions
Abstract
Objective: : We evaluated a new device designed to clean the endotracheal tube in mechanically ventilated patients, the Mucus Shaver.
Design: : Prospective, randomized trial.
Setting: : University hospital intensive care unit.
Patients: : We enrolled 24 patients expected to remain ventilated for >72 hrs.
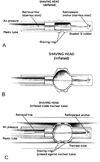
Interventions: : The Mucus Shaver is a concentric inflatable catheter for the removal of mucus and secretions from the interior surface of the endotracheal tube. The Mucus Shaver is advanced to the distal endotracheal tube tip, inflated, and subsequently withdrawn over a period of 3-5 secs. Patients were prospectively randomized within 2 hrs of intubation to receive standard endotracheal tube suctioning treatment or standard suctioning plus Mucus Shaver use until extubation.
Measurements and main results: : During the study period, demographic data, recent medical history, adverse events, and staff evaluation of the Mucus Shaver were recorded. At extubation, each endotracheal tube was removed, cultured, and analyzed by scanning electron microscopy. Twelve patients were assigned to the study group and 12 were assigned to the control group. No adverse events related to the use of the Mucus Shaver were observed. At extubation, only one endotracheal tube from the Mucus Shaver group was colonized, whereas in the control group ten endotracheal tubes were colonized (8% vs. 83%; p < .001). Scanning electron microscopy showed little secretions on the endotracheal tubes from the study group, whereas thick bacterial deposits were present on all the endotracheal tubes from the control group (p < .001 by Fisher exact test, using a maximum biofilm thickness of 30 μm as cut-off). The nursing staff was satisfied by the overall safety, feasibility, and efficacy of the Mucus Shaver.
Conclusions: : The Mucus Shaver is a safe, feasible, and efficient device for endotracheal tube cleaning in the clinical setting. The Mucus Shaver is helpful in preventing endotracheal tube colonization by potentially harmful microorganisms.
Figures


Comment in
-
Keep the tube clean, keep the lungs clean?Crit Care Med. 2012 Jan;40(1):320-1. doi: 10.1097/CCM.0b013e318232d1db. Crit Care Med. 2012. PMID: 22179360 No abstract available.
Similar articles
-
A 72-hour study to test the efficacy and safety of the "Mucus Slurper" in mechanically ventilated sheep.Crit Care Med. 2007 Mar;35(3):906-11. doi: 10.1097/01.CCM.0000257332.62358.0E. Crit Care Med. 2007. PMID: 17255853
-
Intratracheal pulmonary ventilation keeps tracheal tubes clean without impairing mucociliary transport.Scand J Clin Lab Invest. 2002;62(5):351-6. doi: 10.1080/00365510260296500. Scand J Clin Lab Invest. 2002. PMID: 12387580
-
Silver-Coated Endotracheal Tubes Cleaned With a Mechanism for Secretion Removal.Respir Care. 2019 Jan;64(1):1-9. doi: 10.4187/respcare.06222. Epub 2018 Sep 4. Respir Care. 2019. PMID: 30181363 Free PMC article. Clinical Trial.
-
Ventilator associated pneumonia: evolving definitions and preventive strategies.Respir Care. 2013 Jun;58(6):990-1007. doi: 10.4187/respcare.02380. Respir Care. 2013. PMID: 23709196 Review.
-
Microbial composition and antibiotic resistance of biofilms recovered from endotracheal tubes of mechanically ventilated patients.Adv Exp Med Biol. 2015;830:137-55. doi: 10.1007/978-3-319-11038-7_9. Adv Exp Med Biol. 2015. PMID: 25366226 Review.
Cited by
-
Selective digestive decontamination solution used as "lock therapy" prevents and eradicates bacterial biofilm in an in vitro bench-top model.Ann Clin Microbiol Antimicrob. 2020 Sep 23;19(1):44. doi: 10.1186/s12941-020-00387-7. Ann Clin Microbiol Antimicrob. 2020. PMID: 32972419 Free PMC article.
-
Evaluation of a novel endotracheal tube suctioning system incorporating an inflatable sweeper.Can J Respir Ther. 2021 Oct 12;57:138-142. doi: 10.29390/cjrt-2021-026. eCollection 2021. Can J Respir Ther. 2021. PMID: 34734112 Free PMC article.
-
Ex Vivo Evaluation of Secretion-Clearing Device in Reducing Airway Resistance within Endotracheal Tubes.Crit Care Res Pract. 2018 Dec 10;2018:3258396. doi: 10.1155/2018/3258396. eCollection 2018. Crit Care Res Pract. 2018. PMID: 30652032 Free PMC article.
-
Understanding Biofilms and Novel Approaches to the Diagnosis, Prevention, and Treatment of Medical Device-Associated Infections.Infect Dis Clin North Am. 2018 Dec;32(4):915-929. doi: 10.1016/j.idc.2018.06.009. Epub 2018 Sep 18. Infect Dis Clin North Am. 2018. PMID: 30241715 Free PMC article. Review.
-
Assessment of in vivo versus in vitro biofilm formation of clinical methicillin-resistant Staphylococcus aureus isolates from endotracheal tubes.Sci Rep. 2018 Aug 9;8(1):11906. doi: 10.1038/s41598-018-30494-7. Sci Rep. 2018. PMID: 30093624 Free PMC article.
References
-
- Shah C, Kollef MH. Endotracheal tube intraluminal volume loss among mechanically ventilated patients. Crit Care Med. 2004;32:120–125. - PubMed
-
- Villafane MC, Cinnella G, Lofaso F, et al. Gradual reduction of endotracheal tube diameter during mechanical ventilation via different humidification devices. Anesthesiology. 1996;85:1341–1349. - PubMed
-
- Boque MC, Gualis B, Sandiumenge A, et al. Endotracheal tube intraluminal diameter narrowing after mechanical ventilation: use of acoustic reflectometry. Intensive Care Med. 2004;30:2204–2209. - PubMed
-
- Jaber S, Pigeot J, Fodil R, et al. Long-term effects of different humidification systems on endotracheal tube patency: evaluation by the acoustic reflection method. Anesthesiology. 2004;100:782–788. - PubMed
-
- Sottile FD, Marrie TJ, Prough DS, et al. Nosocomial pulmonary infection: possible etiologic significance of bacterial adhesion to endotracheal tubes. Crit Care Med. 1986;14:265–270. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

