A human memory T cell subset with stem cell-like properties
- PMID: 21926977
- PMCID: PMC3192229
- DOI: 10.1038/nm.2446
A human memory T cell subset with stem cell-like properties
Abstract
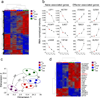
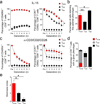
Immunological memory is thought to depend on a stem cell-like, self-renewing population of lymphocytes capable of differentiating into effector cells in response to antigen re-exposure. Here we describe a long-lived human memory T cell population that has an enhanced capacity for self-renewal and a multipotent ability to derive central memory, effector memory and effector T cells. These cells, specific to multiple viral and self-tumor antigens, were found within a CD45RO(-), CCR7(+), CD45RA(+), CD62L(+), CD27(+), CD28(+) and IL-7Rα(+) T cell compartment characteristic of naive T cells. However, they expressed large amounts of CD95, IL-2Rβ, CXCR3, and LFA-1, and showed numerous functional attributes distinctive of memory cells. Compared with known memory populations, these lymphocytes had increased proliferative capacity and more efficiently reconstituted immunodeficient hosts, and they mediated superior antitumor responses in a humanized mouse model. The identification of a human stem cell-like memory T cell population is of direct relevance to the design of vaccines and T cell therapies.
Figures



Comment in
-
Memory in disguise.Nat Med. 2011 Oct 11;17(10):1182-3. doi: 10.1038/nm.2502. Nat Med. 2011. PMID: 21988989 No abstract available.
-
T cell memory: the stem of T cell memory.Nat Rev Immunol. 2011 Oct 25;11(11):716. doi: 10.1038/nri3098. Nat Rev Immunol. 2011. PMID: 22025045 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

