Bcl-xL regulates metabolic efficiency of neurons through interaction with the mitochondrial F1FO ATP synthase
- PMID: 21926988
- PMCID: PMC3186867
- DOI: 10.1038/ncb2330
Bcl-xL regulates metabolic efficiency of neurons through interaction with the mitochondrial F1FO ATP synthase
Erratum in
- Nat Cell Biol. 2011 Nov;13(11):1383
Abstract
Anti-apoptotic Bcl2 family proteins such as Bcl-x(L) protect cells from death by sequestering apoptotic molecules, but also contribute to normal neuronal function. We find in hippocampal neurons that Bcl-x(L) enhances the efficiency of energy metabolism. Our evidence indicates that Bcl-x(L)interacts directly with the β-subunit of the F(1)F(O) ATP synthase, decreasing an ion leak within the F(1)F(O) ATPase complex and thereby increasing net transport of H(+) by F(1)F(O) during F(1)F(O) ATPase activity. By patch clamping submitochondrial vesicles enriched in F(1)F(O) ATP synthase complexes, we find that, in the presence of ATP, pharmacological or genetic inhibition of Bcl-x(L) activity increases the membrane leak conductance. In addition, recombinant Bcl-x(L) protein directly increases the level of ATPase activity of purified synthase complexes, and inhibition of endogenous Bcl-x(L) decreases the level of F(1)F(O) enzymatic activity. Our findings indicate that increased mitochondrial efficiency contributes to the enhanced synaptic efficacy found in Bcl-x(L)-expressing neurons.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

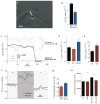

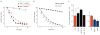
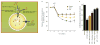

References
-
- Banasiak KJ, Xia Y, Haddad GG. Mechanisms underlying hypoxia-induced neuronal apoptosis. Progress in Neurobiology. 2000;62:215–249. - PubMed
-
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. - PubMed
-
- Fannjiang Y, et al. BAK alters neuronal excitability and can switch from anti- to pro-death function during postnatal development. Developmental Cell. 2003;4:575–585. - PubMed
-
- Kim H, et al. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies.[see comment] Nature Cell Biology. 2006;8:1348–1358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

