Thermal ablation for benign thyroid nodules: radiofrequency and laser
- PMID: 21927553
- PMCID: PMC3168793
- DOI: 10.3348/kjr.2011.12.5.525
Thermal ablation for benign thyroid nodules: radiofrequency and laser
Abstract
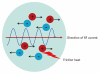
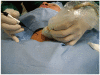
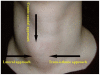
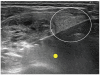
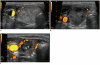
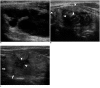
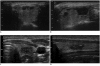
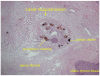
Although ethanol ablation has been successfully used to treat cystic thyroid nodules, this procedure is less effective when the thyroid nodules are solid. Radiofrequency (RF) ablation, a newer procedure used to treat malignant liver tumors, has been valuable in the treatment of benign thyroid nodules regardless of the extent of the solid component. This article reviews the basic physics, techniques, applications, results, and complications of thyroid RF ablation, in comparison to laser ablation.
Keywords: Ethanol ablation; Laser ablation; Radiofrequency ablation; Thyroid nodule; Ultrasonography.
Figures



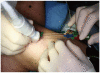

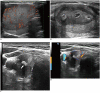
References
-
- Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993;328:553–559. - PubMed
-
- Jeong WK, Baek JH, Rhim H, Kim YS, Kwak MS, Jeong HJ, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol. 2008;18:1244–1250. - PubMed
-
- Papini E, Guglielmi R, Bizzarri G, Pacella CM. Ultrasound-guided laser thermal ablation for treatment of benign thyroid nodules. Endocr Pract. 2004;10:276–283. - PubMed
-
- Shemen LJ, Strong EW. Complications after total thyroidectomy. Otolaryngol Head Neck Surg. 1989;101:472–475. - PubMed
-
- Papini E, Guglielmi R, Bizzarri G, Graziano F, Bianchini A, Brufani C, et al. Treatment of benign cold thyroid nodules: a randomized clinical trial of percutaneous laser ablation versus levothyroxine therapy or follow-up. Thyroid. 2007;17:229–235. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

