Diverse roles of TGF-β/Smads in renal fibrosis and inflammation
- PMID: 21927575
- PMCID: PMC3174390
- DOI: 10.7150/ijbs.7.1056
Diverse roles of TGF-β/Smads in renal fibrosis and inflammation
Abstract
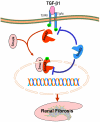
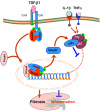
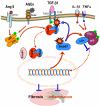
TGF-β1 has been long considered as a key mediator in renal fibrosis and induces renal scarring largely by activating its downstream Smad signaling pathway. Interestingly, while mice overexpressing active TGF-β1 develop progressive renal injury, latent TGF-β1 plays a protective role in renal fibrosis and inflammation. Under disease conditions, Smad2 and Smad3 are highly activated, while Smad7 is degraded through the ubiquitin proteasome degradation mechanism. In addition to TGF-β1, many pathogenic mediators such as angiotensin II and advanced glycation end products can also activate the Smad pathway via both TGF-β-dependent and independent mechanisms. Smads interact with other signaling pathways, such as the MAPK and NF-κB pathways, to positively or negatively regulate renal inflammation and fibrosis. Studies from gene knockout mice demonstrate that TGF-β1 acts by stimulating its downstream Smads to diversely regulate kidney injury. In the context of renal fibrosis and inflammation, Smad3 is pathogenic, while Smad2 and Smad7 are protective. Smad4 exerts its diverse roles by transcriptionally enhancing Smad3-mediated renal fibrosis while inhibiting NF-κB-driven renal inflammation via a Smad7-dependent mechanism. Furthermore, we also demonstrated that TGF-β1 acts by stimulating Smad3 to positively or negatively regulate microRNAs to exert its fibrotic role in kidney disease. In conclusion, TGF-β/Smad signaling is a major pathway leading to kidney disease. Smad3 is a key mediator in renal fibrosis and inflammation, whereas Smad2 and Smad7 are renoprotective. Smad4 exerts its diverse role in promoting renal fibrosis while inhibiting inflammation. Thus, targeting the downstream TGF-β/Smad3 signaling pathway by gene transfer of either Smad7 or Smad3-dependent microRNAs may represent a specific and effective therapeutic strategy for kidney disease.
Keywords: TGF-β/Smads; anti-TGF-β therapy; fibrosis; inflammation; microRNAs..
Conflict of interest statement
Conflict of Interests: The author has declared that no conflict of interest exists.
Figures


References
-
- Eddy AA, Neilson EG. Chronic kidney disease progression. J Am Soc Nephrol. 2006;17:2964–66. - PubMed
-
- Bottinger EP. TGF-beta in renal injury and disease. Semin Nephrol. 2007;27:309–20. - PubMed
-
- Wang W, Koka V, Lan HY. Transforming growth factor-beta and Smad signalling in kidney diseases. Nephrology (Carlton) 2005 Feb;10(1):48–56. - PubMed
-
- Roberts AB. Molecular and cell biology of TGF-beta. Miner Electrolyte Metab. 1998;24:111–9. - PubMed
-
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGFbeta family signalling. Nature. 2003;425:577–84. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

