Intracellular events and cell fate in filovirus infection
- PMID: 21927676
- PMCID: PMC3172725
- DOI: 10.3390/v3081501
Intracellular events and cell fate in filovirus infection
Abstract
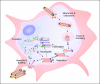
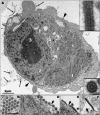
Marburg and Ebola viruses cause a severe hemorrhagic disease in humans with high fatality rates. Early target cells of filoviruses are monocytes, macrophages, and dendritic cells. The infection spreads to the liver, spleen and later other organs by blood and lymph flow. A hallmark of filovirus infection is the depletion of non-infected lymphocytes; however, the molecular mechanisms leading to the observed bystander lymphocyte apoptosis are poorly understood. Also, there is limited knowledge about the fate of infected cells in filovirus disease. In this review we will explore what is known about the intracellular events leading to virus amplification and cell damage in filovirus infection. Furthermore, we will discuss how cellular dysfunction and cell death may correlate with disease pathogenesis.
Keywords: Ebola Virus; Marburg Virus; animal models; bystander apoptosis; cell death; filoviruses; target cells; ultrastructural analysis; viral replication cycle; virus-cell interaction.
Figures


References
-
- Kuhn JH. Filoviruses. A compendium of 40 years of epidemiological, clinical, and laboratory studies. Arch Virol Suppl. 2008;20:13–360. - PubMed
-
- Mohamadzadeh M, Chen L, Schmaljohn AL. How Ebola and Marburg viruses battle the immune system. Nat Rev Immunol. 2007;7:556–567. - PubMed
-
- Bradfute SB, Swanson PE, Smith MA, Watanabe E, McDunn JE, Hotchkiss RS, Bavari S. Mechanisms and consequences of ebolavirus-induced lymphocyte apoptosis. J Immunol. 2010;184:327–335. - PubMed
-
- Ksiazek TG, West CP, Rollin PE, Jahrling PB, Peters CJ. ELISA for the detection of antibodies to Ebola viruses. J Infect Dis. 1999;179:S192–S198. - PubMed
-
- Onyango CO, Opoka ML, Ksiazek TG, Formenty P, Ahmed A, Tukei PM, Sang RC, Ofula VO, Konongoi SL, Coldren RL, et al. Laboratory diagnosis of Ebola hemorrhagic fever during an outbreak in Yambio, Sudan, 2004. J Infect Dis. 2007;196:S193–S198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

