Novel bulking agent for faecal incontinence
- PMID: 21928378
- PMCID: PMC3229845
- DOI: 10.1002/bjs.7699
Novel bulking agent for faecal incontinence
Abstract
Background: Various injectable bulking agents have been used for the treatment of faecal incontinence (FI). However, encouraging early results are not maintained over time. This study aimed to assess short- and medium-term results of a new bulking agent for the treatment of FI.
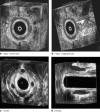
Methods: The Gatekeeper(™) prosthesis comprises a thin solid polyacrylonitrile cylinder that becomes thicker, shorter and softer within 24 h after implantation. Fourteen patients with FI underwent treatment with Gatekeeper(™) under local anaesthesia. Four prostheses were implanted in the intersphincteric space in each patient, under endoanal ultrasound guidance. Number of episodes of major FI, Cleveland Clinic FI score (CCFIS), Vaizey score, anorectal manometry, endoanal ultrasonography (EUS), health status and quality of life (Short Form 36 and Faecal Incontinence Quality of Life questionnaires) were assessed before and after treatment.
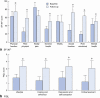
Results: Mean(s.d.) follow-up was 33·5(12·4) months. There were no complications. There was a significant decrease in major FI episodes from 7·1(7·4) per week at baseline to 1·4(4·0), 1·0(3·2) and 0·4(0·6) per week respectively at 1-month, 3-month and last follow-up (P = 0·002). CCFIS improved significantly from 12·7(3·3) to 4·1(3·0), 3·9(2·6) and 5·1(3·0) respectively (P < 0·001), and Vaizey score from 15·4(3·3) to 7·1(3·9), 4·7(3·0) and 6·9(5·0) respectively (P = 0·010). Soiling and ability to postpone defaecation improved significantly, and patients reported significant improvement in health status and quality of life. At follow-up, manometric parameters had not changed and EUS did not demonstrate any prosthesis dislocation.
Conclusion: The Gatekeeper(™) anal implant seemed safe, reliable and effective. Initial clinical improvement was maintained over time, and follow-up data were encouraging.
Copyright © 2011 British Journal of Surgery Society Ltd. Published by John Wiley & Sons, Ltd.
Figures

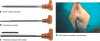


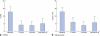
Comment in
-
Novel bulking agent for faecal incontinence (Br J Surg 2011; 98: 1644-1652).Br J Surg. 2011 Nov;98(11):1653. doi: 10.1002/bjs.7746. Br J Surg. 2011. PMID: 21964687 No abstract available.
References
-
- Shafik A. Polytetrafluoroethylene injection for the treatment of partial fecal incontinence. Int Surg. 1993;78:159–161. - PubMed
-
- Shafik A. Perianal injection of autologous fat for treatment of sphincteric incontinence. Dis Colon Rectum. 1995;38:583–587. - PubMed
-
- Kumar D, Benson MJ, Bland JE. Glutaraldehyde cross-linked collagen in the treatment of faecal incontinence. Br J Surg. 1998;85:978–979. - PubMed
-
- Malouf AJ, Vaizey CJ, Norton CS, Kamm MA. Internal anal sphincter augmentation for fecal incontinence using injectable silicone biomaterial. Dis Colon Rectum. 2001;44:595–600. - PubMed
-
- Davis K, Kumar D, Poloniecki J. Preliminary evaluation of an injectable anal sphincter bulking agent (Durasphere) in the management of faecal incontinence. Aliment Pharmacol Ther. 2003;18:237–243. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

