A dedicated cone-beam CT system for musculoskeletal extremities imaging: design, optimization, and initial performance characterization
- PMID: 21928644
- PMCID: PMC3172864
- DOI: 10.1118/1.3611039
A dedicated cone-beam CT system for musculoskeletal extremities imaging: design, optimization, and initial performance characterization
Abstract
Purpose: This paper reports on the design and initial imaging performance of a dedicated cone-beam CT (CBCT) system for musculoskeletal (MSK) extremities. The system complements conventional CT and MR and offers a variety of potential clinical and logistical advantages that are likely to be of benefit to diagnosis, treatment planning, and assessment of therapy response in MSK radiology, orthopaedic surgery, and rheumatology.
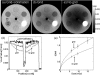
Methods: The scanner design incorporated a host of clinical requirements (e.g., ability to scan the weight-bearing knee in a natural stance) and was guided by theoretical and experimental analysis of image quality and dose. Such criteria identified the following basic scanner components and system configuration: a flat-panel detector (FPD, Varian 3030+, 0.194 mm pixels); and a low-power, fixed anode x-ray source with 0.5 mm focal spot (SourceRay XRS-125-7K-P, 0.875 kW) mounted on a retractable C-arm allowing for two scanning orientations with the capability for side entry, viz. a standing configuration for imaging of weight-bearing lower extremities and a sitting configuration for imaging of tensioned upper extremity and unloaded lower extremity. Theoretical modeling employed cascaded systems analysis of modulation transfer function (MTF) and detective quantum efficiency (DQE) computed as a function of system geometry, kVp and filtration, dose, source power, etc. Physical experimentation utilized an imaging bench simulating the scanner geometry for verification of theoretical results and investigation of other factors, such as antiscatter grid selection and 3D image quality in phantom and cadaver, including qualitative comparison to conventional CT.
Results: Theoretical modeling and benchtop experimentation confirmed the basic suitability of the FPD and x-ray source mentioned above. Clinical requirements combined with analysis of MTF and DQE yielded the following system geometry: a -55 cm source-to-detector distance; 1.3 magnification; a 20 cm diameter bore (20 x 20 x 20 cm3 field of view); total acquisition arc of -240 degrees. The system MTF declines to 50% at -1.3 mm(-1) and to 10% at -2.7 mm(-1), consistent with sub-millimeter spatial resolution. Analysis of DQE suggested a nominal technique of 90 kVp (+0.3 mm Cu added filtration) to provide high imaging performance from -500 projections at less than -0.5 kW power, implying -6.4 mGy (0.064 mSv) for low-dose protocols and -15 mGy (0.15 mSv) for high-quality protocols. The experimental studies show improved image uniformity and contrast-to-noise ratio (without increase in dose) through incorporation of a custom 10:1 GR antiscatter grid. Cadaver images demonstrate exquisite bone detail, visualization of articular morphology, and soft-tissue visibility comparable to diagnostic CT (10-20 HU contrast resolution).
Conclusions: The results indicate that the proposed system will deliver volumetric images of the extremities with soft-tissue contrast resolution comparable to diagnostic CT and improved spatial resolution at potentially reduced dose. Cascaded systems analysis provided a useful basis for system design and optimization without costly repeated experimentation. A combined process of design specification, image quality analysis, clinical feedback, and revision yielded a prototype that is now awaiting clinical pilot studies. Potential advantages of the proposed system include reduced space and cost, imaging of load-bearing extremities, and combined volumetric imaging with real-time fluoroscopy and digital radiography.
Figures









References
-
- Geijer M., Börjesson A., and Göthlin J., “Clinical utility of tomosynthesis in suspected scaphoid fracture. A pilot study,” Skeletal Radiology, 40(7), 863–867 (2011). - PubMed
-
- Siewerdsen J. H., Moseley D. J., Burch S., Bisland S. K., Bogaards A., Wilson B. C., and Jaffray D. A., “Volume CT with a flat-panel detector on a mobile, isocentric C-arm: Pre-clinical investigation in guidance of minimally invasive surgery,” Med. Phys. 32, 241–254 (2005).10.1118/1.1836331 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

