Multiple artificial microRNAs targeting conserved motifs of the replicase gene confer robust transgenic resistance to negative-sense single-stranded RNA plant virus
- PMID: 21929564
- PMCID: PMC6638711
- DOI: 10.1111/j.1364-3703.2011.00747.x
Multiple artificial microRNAs targeting conserved motifs of the replicase gene confer robust transgenic resistance to negative-sense single-stranded RNA plant virus
Abstract
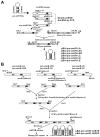
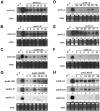
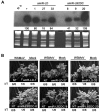
MicroRNAs (miRNAs) regulate the abundance of target mRNAs by guiding cleavage at sequence complementary regions. In this study, artificial miRNAs (amiRNAs) targeting conserved motifs of the L (replicase) gene of Watermelon silver mottle virus (WSMoV) were constructed using Arabidopsis pre-miRNA159a as the backbone. The constructs included six single amiRNAs targeting motifs A, B1, B2, C, D of E, and two triple amiRNAs targeting motifs AB1E or B2DC. Processing of pre-amiRNAs was confirmed by agro-infiltration, and transgenic Nicotiana benthamiana plants expressing each amiRNA were generated. Single amiRNA transgenic lines expressing amiR-LB2 or amiR-LD showed resistance to WSMoV by delaying symptom development. Triple amiRNA lines expressing amiR-LB2, amiR-LD and amiR-LC provided complete resistance against WSMoV, with no indication of infection 28 days after inoculation. Resistance levels were positively correlated with amiRNA expression levels in these single and triple amiRNA lines. The triple amiR-LAB1E line did not provide resistance to WSMoV. Similarly, the poorly expressed amiR-LC and amiR-LE lines did not provide resistance to WSMoV. The amiR-LA- and amiR-LB1-expressing lines were susceptible to WSMoV, and their additional susceptibility to the heterologous Turnip mosaic virus harbouring individual target sequences indicated that these two amiRNAs have no effect in vivo. Transgenic lines expressing amiR-LB2 exhibited delayed symptoms after challenge with Peanut bud necrosis virus having a single mismatch in the target site. Overall, our results indicate that two amiRNAs, amiR-LB2 and amiR-LD, of the six designed amiRNAs confer moderate resistance against WSMoV, and the triple construct including the two amiRNAs provides complete resistance.
© 2011 THE AUTHORS. MOLECULAR PLANT PATHOLOGY © 2011 BSPP AND BLACKWELL PUBLISHING LTD.
Figures




References
-
- Ai, T. , Zhang, L. , Gao, Z. , Zhu, C.X. and Guo, X. (2011) Highly efficient virus resistance mediated by artificial microRNAs that target the suppressor of PVX and PVY in plants. Plant Biol. 13, 304–316. - PubMed
-
- Bartel, D.P. (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell, 116, 281–297. - PubMed
-
- Baulcombe, D. (2004) RNA silencing in plants. Nature, 431, 356–363. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

