Dialysis cannot be dosed
- PMID: 21929590
- PMCID: PMC4124940
- DOI: 10.1111/j.1525-139X.2011.00979.x
Dialysis cannot be dosed
Abstract
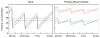
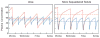
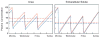
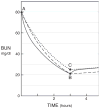
Adequate dialysis is difficult to define because we have not identified the toxic solutes that contribute most to uremic illness. Dialysis prescriptions therefore cannot be adjusted to control the levels of these solutes. The current solution to this problem is to define an adequate dose of dialysis on the basis of fraction of urea removed from the body. This has provided a practical guide to treatment as the dialysis population has grown over the past 25 years. Indeed, a lower limit to Kt/V(urea) (or the related urea reduction ratio) is now established as a quality indicator by the Centers for Medicare and Medicaid for chronic hemodialysis patients in the United States. For the present, this urea-based standard provides a useful tool to avoid grossly inadequate dialysis. Dialysis dosing, however, based on measurement of a single, relatively nontoxic solute can provide only a very limited guide toward improved treatment. Prescriptions which have similar effects on the index solute can have widely different effects on other solutes. The dose concept discourages attempts to increase the removal of such solutes independent of the index solute. The dose concept further assumes that important solutes are produced at a constant rate relative to body size, and discourages attempts to augment dialysis treatment by reducing solute production. Identification of toxic solutes would provide a more rational basis for the prescription of dialysis and ultimately for improved treatment of patients with renal failure.
Published 2011. This article is a U.S. Government work and is in the public domain in the USA.
Conflict of interest statement
The authors have no financial interests to disclose.
Figures
References
-
- Hemodialysis Adequacy 2006 Work Group. Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis. 2006;48(Suppl 1):S2–S90. - PubMed
-
- Depner TA. Prescribing Hemodialysis: A Guide to Urea Modeling. Norwall, MA: Kluwer Academic Publishers; 1991.
-
- Lowrie EG, Laird NM, Parker TF, Sargent JA. Effect of the hemodialysis prescription of patient morbidity: report from the National Cooperative Dialysis Study. N Engl J Med. 1981;305:1176–1181. - PubMed
-
- Burton BT. Adequacy of dialysis. Introduction: purpose of the conference. Kidney Int. 1975;7(Suppl 2):S1. - PubMed
-
- Teschan PE. Electroencephalographic and other neurophysiological abnormalities in uremia. Kidney Int. 1975;7(Suppl 2):S210–S216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

