Stimulant reduction intervention using dosed exercise (STRIDE) - CTN 0037: study protocol for a randomized controlled trial
- PMID: 21929768
- PMCID: PMC3191354
- DOI: 10.1186/1745-6215-12-206
Stimulant reduction intervention using dosed exercise (STRIDE) - CTN 0037: study protocol for a randomized controlled trial
Abstract
Background: There is a need for novel approaches to the treatment of stimulant abuse and dependence. Clinical data examining the use of exercise as a treatment for the abuse of nicotine, alcohol, and other substances suggest that exercise may be a beneficial treatment for stimulant abuse, with direct effects on decreased use and craving. In addition, exercise has the potential to improve other health domains that may be adversely affected by stimulant use or its treatment, such as sleep disturbance, cognitive function, mood, weight gain, quality of life, and anhedonia, since it has been shown to improve many of these domains in a number of other clinical disorders. Furthermore, neurobiological evidence provides plausible mechanisms by which exercise could positively affect treatment outcomes. The current manuscript presents the rationale, design considerations, and study design of the National Institute on Drug Abuse (NIDA) Clinical Trials Network (CTN) CTN-0037 Stimulant Reduction Intervention using Dosed Exercise (STRIDE) study.
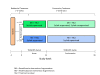
Methods/design: STRIDE is a multisite randomized clinical trial that compares exercise to health education as potential treatments for stimulant abuse or dependence. This study will evaluate individuals diagnosed with stimulant abuse or dependence who are receiving treatment in a residential setting. Three hundred and thirty eligible and interested participants who provide informed consent will be randomized to one of two treatment arms: Vigorous Intensity High Dose Exercise Augmentation (DEI) or Health Education Intervention Augmentation (HEI). Both groups will receive TAU (i.e., usual care). The treatment arms are structured such that the quantity of visits is similar to allow for equivalent contact between groups. In both arms, participants will begin with supervised sessions 3 times per week during the 12-week acute phase of the study. Supervised sessions will be conducted as one-on-one (i.e., individual) sessions, although other participants may be exercising at the same time. Following the 12-week acute phase, participants will begin a 6-month continuation phase during which time they will attend one weekly supervised DEI or HEI session.
Clinical trials registry: ClinicalTrials.gov, NCT01141608 http://clinicaltrials.gov/ct2/show/NCT01141608?term=Stimulant+Reduction+Intervention+using+Dosed+Exercise&rank=1.
References
-
- Ball SA, Martino S, Nich C, Frankforter TL, Van Horn D, Crits-Christoph P, Woody GE, Obert JL, Farentinos C, Carroll KM. Site matters: Multisite randomized trial of motivational enhancement therapy in community drug abuse clinics. Journal of Consulting and Clinical Psychology. 2007;75:556–567. - PMC - PubMed
-
- Wetzel H, Szegedi A, Scheurich A, Lorch B, Schlafke D, Sittinger H, Wobrock T, Muller MJ, Anghelescu I, Hautzinger M. NeVeR Study Group. Combination treatment with nefazodone and cognitive-behavioral therapy for relapse prevention in alcohol-dependent men: A randomized controlled study. Journal of Clinical Psychiatry. 2004;65:1406–1413. doi: 10.4088/JCP.v65n1017. - DOI - PubMed