Gap junctions and other mechanisms of cell-cell communication regulate basal insulin secretion in the pancreatic islet
- PMID: 21930600
- PMCID: PMC3240884
- DOI: 10.1113/jphysiol.2011.218909
Gap junctions and other mechanisms of cell-cell communication regulate basal insulin secretion in the pancreatic islet
Abstract
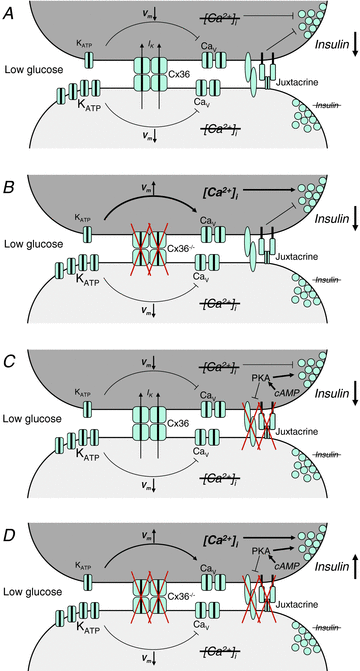
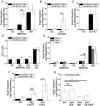
Cell-cell communication in the islet of Langerhans is important for the regulation of insulin secretion. Gap-junctions coordinate oscillations in intracellular free-calcium ([Ca(2+)](i)) and insulin secretion in the islet following elevated glucose. Gap-junctions can also ensure that oscillatory [Ca(2+)](i) ceases when glucose is at a basal levels. We determine the roles of gap-junctions and other cell-cell communication pathways in the suppression of insulin secretion under basal conditions. Metabolic, electrical and insulin secretion levels were measured from islets lacking gap-junction coupling following deletion of connexion36 (Cx36(-/-)), and these results were compared to those obtained using fully isolated β-cells. K(ATP) loss-of-function islets provide a further experimental model to specifically study gap-junction mediated suppression of electrical activity. In isolated β-cells or Cx36(-/-) islets, elevations in [Ca(2+)](i) persisted in a subset of cells even at basal glucose. Isolated β-cells showed elevated insulin secretion at basal glucose; however, insulin secretion from Cx36(-/-) islets was minimally altered. [Ca(2+)](i) was further elevated under basal conditions, but insulin release still suppressed in K(ATP) loss-of-function islets. Forced elevation of cAMP led to PKA-mediated increases in insulin secretion from islets lacking gap-junctions, but not from islets expressing Cx36 gap junctions. We conclude there is a redundancy in how cell-cell communication in the islet suppresses insulin release. Gap junctions suppress cellular heterogeneity and spontaneous [Ca(2+)](i) signals, while other juxtacrine mechanisms, regulated by PKA and glucose, suppress more distal steps in exocytosis. Each mechanism is sufficiently robust to compensate for a loss of the other and still suppress basal insulin secretion.
Figures


References
-
- Asa SL, Tannenbaum GS. Cell-cell communication in the pituitary: orchestrator of pulsatile growth hormone secretion? Trends Endocrinol Metab. 2006;17:299–300. - PubMed
-
- Bennett BD, Jetton TL, Ying GT, Magnuson MA, Piston DW. Quantitative subcellular imaging of glucose metabolism within intact pancreatic islets. J Biol Chem. 1996;271:3647–3651. - PubMed
-
- Bonnefont X, Lacampagne A, Sanchez-Hormigo A, Fino E, Creff A, Mathieu MN, Smallwood S, Carmignac D, Fontanaud P, Travo P, Alonso G, Courtois-Coutry N, Pincus SM, Robinson I, Mollard P. Revealing the large-scale network organization of growth hormone-secreting cells. Proc Natl Acad Sci U S A. 2005;102:16880–16885. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K99-DK085145/DK/NIDDK NIH HHS/United States
- P60 DK020593/DK/NIDDK NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- K99 DK085145/DK/NIDDK NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- R00 DK085145/DK/NIDDK NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- R01-DK053434/DK/NIDDK NIH HHS/United States
- R01 DK053434/DK/NIDDK NIH HHS/United States
- CA68485/CA/NCI NIH HHS/United States
- R01-DK46409/DK/NIDDK NIH HHS/United States
- DK58404/DK/NIDDK NIH HHS/United States
- R01 DK046409/DK/NIDDK NIH HHS/United States
- DK20593/DK/NIDDK NIH HHS/United States
- P20-GM072048/GM/NIGMS NIH HHS/United States
- P20 GM072048/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

