Adenylate cyclase 1 promotes strengthening and experience-dependent plasticity of whisker relay synapses in the thalamus
- PMID: 21930601
- PMCID: PMC3249040
- DOI: 10.1113/jphysiol.2011.213702
Adenylate cyclase 1 promotes strengthening and experience-dependent plasticity of whisker relay synapses in the thalamus
Abstract
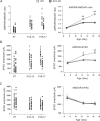
Synaptic refinement, a process that involves elimination and strengthening of immature synapses, is critical for the development of neural circuits and behaviour. The present study investigates the role of adenylate cyclase 1 (AC1) in developmental refinement of excitatory synapses in the thalamus at the single-cell level. In the mouse, thalamic relay synapses of the lemniscal pathway undergo extensive remodelling during the second week after birth, and AC1 is highly expressed in both pre- and postsynaptic neurons during this period. Synaptic connectivity was analysed by patch-clamp recording in acute slices obtained from mice carrying a targeted null mutation of the adenylate cyclase 1 gene (AC1-KO) and wild-type littermates. We found that deletion of AC1 had no effect on the number of relay inputs received by thalamic neurons during development. In contrast, there was a selective reduction of AMPA-receptor-mediated synaptic responses in mutant thalamic neurons, and the effect increased with age. Furthermore, experience-dependent plasticity was impaired in thalamic neurons of AC1-KO mice. Whisker deprivation during early life altered the number and properties of relay inputs received by thalamic neurons in wild-type mice, but had no effects in AC1-KO mice. Our findings underline a role for AC1 in experience-dependent plasticity of excitatory synapses.
Figures




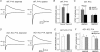
Comment in
-
AC-1 and synaptic development.J Physiol. 2012 Jan 1;590(1):3. doi: 10.1113/jphysiol.2011.223396. J Physiol. 2012. PMID: 22210283 Free PMC article. No abstract available.
Similar articles
-
Elimination of redundant synaptic inputs in the absence of synaptic strengthening.J Neurosci. 2011 Nov 16;31(46):16675-84. doi: 10.1523/JNEUROSCI.4569-11.2011. J Neurosci. 2011. PMID: 22090494 Free PMC article.
-
Developmental remodelling of the lemniscal synapse in the ventral basal thalamus of the mouse.J Physiol. 2006 May 15;573(Pt 1):121-32. doi: 10.1113/jphysiol.2006.106542. Epub 2006 Mar 31. J Physiol. 2006. PMID: 16581865 Free PMC article.
-
A critical window for experience-dependent plasticity at whisker sensory relay synapse in the thalamus.J Neurosci. 2008 Dec 10;28(50):13621-8. doi: 10.1523/JNEUROSCI.4785-08.2008. J Neurosci. 2008. PMID: 19074025 Free PMC article.
-
Sensory Activity-Dependent and Sensory Activity-Independent Properties of the Developing Rodent Trigeminal Principal Nucleus.Dev Neurosci. 2016;38(3):163-170. doi: 10.1159/000446395. Epub 2016 Jun 9. Dev Neurosci. 2016. PMID: 27287019 Free PMC article. Review.
-
Spike timing and synaptic dynamics at the awake thalamocortical synapse.Prog Brain Res. 2005;149:91-105. doi: 10.1016/S0079-6123(05)49008-1. Prog Brain Res. 2005. PMID: 16226579 Review.
Cited by
-
A Selective Adenylyl Cyclase 1 Inhibitor Relieves Pain Without Causing Tolerance.Front Pharmacol. 2022 Jul 11;13:935588. doi: 10.3389/fphar.2022.935588. eCollection 2022. Front Pharmacol. 2022. PMID: 35899113 Free PMC article.
-
The metabotropic glutamate receptor subtype 1 regulates development and maintenance of lemniscal synaptic connectivity in the somatosensory thalamus.PLoS One. 2019 Dec 27;14(12):e0226820. doi: 10.1371/journal.pone.0226820. eCollection 2019. PLoS One. 2019. PMID: 31881077 Free PMC article.
-
Essential role of postsynaptic NMDA receptors in developmental refinement of excitatory synapses.Proc Natl Acad Sci U S A. 2013 Jan 15;110(3):1095-100. doi: 10.1073/pnas.1212971110. Epub 2012 Dec 31. Proc Natl Acad Sci U S A. 2013. PMID: 23277569 Free PMC article.
-
Adenylyl Cyclases as Therapeutic Targets in Neuroregeneration.Int J Mol Sci. 2025 Jun 25;26(13):6081. doi: 10.3390/ijms26136081. Int J Mol Sci. 2025. PMID: 40649859 Free PMC article. Review.
-
AC-1 and synaptic development.J Physiol. 2012 Jan 1;590(1):3. doi: 10.1113/jphysiol.2011.223396. J Physiol. 2012. PMID: 22210283 Free PMC article. No abstract available.
References
-
- Abdel-Majid RM, Leong WL, Schalkwyk LC, Smallman DS, Wong ST, Storm DR, Fine A, Dobson MJ, Guernsey DL, Neumann PE. Loss of adenylyl cyclase I activity disrupts patterning of mouse somatosensory cortex. Nat Genet. 1998;19:289–291. - PubMed
-
- Allen CB, Celikel T, Feldman DE. Long-term depression induced by sensory deprivation during cortical map plasticityin vivo. Nat Neurosci. 2003;6:291–299. - PubMed
-
- Chung HJ, Steinberg JP, Huganir RL, Linden DJ. Requirement of AMPA receptor GluR2 phosphorylation for cerebellar long-term depression. Science. 2003;300:1751–1755. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

