Induction of lectin-like transcript 1 (LLT1) protein cell surface expression by pathogens and interferon-γ contributes to modulate immune responses
- PMID: 21930700
- PMCID: PMC3207416
- DOI: 10.1074/jbc.M111.285312
Induction of lectin-like transcript 1 (LLT1) protein cell surface expression by pathogens and interferon-γ contributes to modulate immune responses
Abstract
CD161 is a C-type lectin-like receptor expressed on human natural killer (NK) cells and subsets of T cells. CD161 has been described as an inhibitory receptor that regulates NK cell-mediated cytotoxicity and IFN-γ production. Its role on T cells has remained unclear. Studies have shown that triggering of CD161 enhances NK T cell proliferation and T cell-IFN-γ production while inhibiting TNF-α production by CD8(+) T cells. Lectin-like transcript 1 (LLT1), the ligand of CD161, was found to be expressed on Toll-like receptor (TLR)-activated plasmacytoid and monocyte-derived dendritic cells (DC) and on activated B cells. Using newly developed anti-LLT1 mAbs, we show that LLT1 is not expressed on the surface of circulating B and T lymphocytes, NK cells, monocytes, and dendritic cells but that LLT1 is up-regulated upon activation. Not only TLR-stimulated dendritic cells and B cells but also T cell receptor-activated T cells and activated NK cells up-regulate LLT1. Interestingly, IFN-γ increases LLT1 expression level on antigen-presenting cells. LLT1 is also induced on B cells upon viral infection such as Epstein-Barr virus or HIV infection and in inflamed tonsils. Finally, expression of LLT1 on B cells inhibits NK cell function but costimulates T cell proliferation or IFN-γ production, and coengagement of CD161 with CD3 increases IL-17 secretion. Altogether, our results point toward a role for LLT1/CD161 in modulating immune responses to pathogens.
Figures
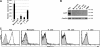

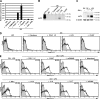
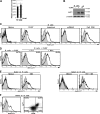
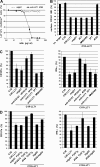

References
-
- Palm N. W., Medzhitov R. (2009) Immunol. Rev. 227, 221–233 - PubMed
-
- Lanier L. L., Chang C., Phillips J. H. (1994) J. Immunol. 153, 2417–2428 - PubMed
-
- Poggi A., Zocchi M. R., Costa P., Ferrero E., Borsellino G., Placido R., Galgani S., Salvetti M., Gasperini C., Ristori G., Brosnan C. F., Battistini L. (1999) J. Immunol. 162, 4349–4354 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

