Ectonucleotide pyrophosphatase/phosphodiesterase-1 (ENPP1) protein regulates osteoblast differentiation
- PMID: 21930712
- PMCID: PMC3234731
- DOI: 10.1074/jbc.M111.221689
Ectonucleotide pyrophosphatase/phosphodiesterase-1 (ENPP1) protein regulates osteoblast differentiation
Abstract
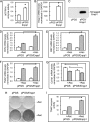
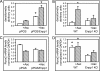
ENPP1 (ectonucleotide pyrophosphatase/phosphodiesterase-1) is an established regulator of tissue mineralization. Previous studies demonstrated that ENPP1 is expressed in differentiated osteoblasts and that ENPP1 influences matrix mineralization by increasing extracellular levels of inorganic pyrophosphate. ENPP1 is also expressed in osteoblastic precursor cells when stimulated with FGF2, but the role of ENPP1 in preosteoblastic and other precursor cells is unknown. Here we investigate the function of ENPP1 in preosteoblasts. We find that ENPP1 expression is critical for osteoblastic differentiation and that this effect is not mediated by changes in extracellular concentration levels of phosphate or pyrophosphate or ENPP1 catalytic activity. MC3T3E1(C4) preosteoblastic cells, in which ENPP1 expression was suppressed by ENPP1-specific shRNA, and calvarial cells isolated from Enpp1 knock-out mice show defective osteoblastic differentiation upon stimulation with ascorbate, as indicated by a lack of cellular morphological change, a lack of osteoblast marker gene expression, and an inability to mineralize matrix. Additionally, MC3T3E1(C4) cells, in which wild type or catalytic inactive ENPP1 expression was increased, exhibited an increased tendency to differentiate, as evidenced by increased osteoblast marker gene expression and increased mineralization. Notably, treatment of cells with inorganic phosphate or pyrophosphate inhibited, as opposed to enhanced, expression of multiple genes that are expressed in association with osteoblast differentiation, matrix deposition, and mineralization. Our results indicate that ENPP1 plays multiple and distinct roles in the development of mineralized tissues and that the influence of ENPP1 on osteoblast differentiation and gene expression may include a mechanism that is independent of its catalytic activity.
Figures







Similar articles
-
FGF2 alters expression of the pyrophosphate/phosphate regulating proteins, PC-1, ANK and TNAP, in the calvarial osteoblastic cell line, MC3T3E1(C4).Connect Tissue Res. 2005;46(4-5):184-92. doi: 10.1080/03008200500237203. Connect Tissue Res. 2005. PMID: 16546821
-
Concerted regulation of inorganic pyrophosphate and osteopontin by akp2, enpp1, and ank: an integrated model of the pathogenesis of mineralization disorders.Am J Pathol. 2004 Apr;164(4):1199-209. doi: 10.1016/S0002-9440(10)63208-7. Am J Pathol. 2004. PMID: 15039209 Free PMC article.
-
1,25-Dihydroxyvitamin D3 and extracellular calcium promote mineral deposition via NPP1 activity in a mature osteoblast cell line MLO-A5.Mol Cell Endocrinol. 2015 Sep 5;412:140-7. doi: 10.1016/j.mce.2015.06.005. Epub 2015 Jun 6. Mol Cell Endocrinol. 2015. PMID: 26054750
-
ENPP1 in Blood and Bone: Skeletal and Soft Tissue Diseases Induced by ENPP1 Deficiency.Annu Rev Pathol. 2024 Jan 24;19:507-540. doi: 10.1146/annurev-pathmechdis-051222-121126. Epub 2023 Oct 23. Annu Rev Pathol. 2024. PMID: 37871131 Free PMC article. Review.
-
ENPP1, an Old Enzyme with New Functions, and Small Molecule Inhibitors-A STING in the Tale of ENPP1.Molecules. 2019 Nov 19;24(22):4192. doi: 10.3390/molecules24224192. Molecules. 2019. PMID: 31752288 Free PMC article. Review.
Cited by
-
ATP-degrading ENPP1 is required for survival (or persistence) of long-lived plasma cells.Sci Rep. 2017 Dec 19;7(1):17867. doi: 10.1038/s41598-017-18028-z. Sci Rep. 2017. PMID: 29259245 Free PMC article.
-
Enzyme replacement for craniofacial skeletal defects and craniosynostosis in murine hypophosphatasia.Bone. 2015 Sep;78:203-11. doi: 10.1016/j.bone.2015.05.005. Epub 2015 May 8. Bone. 2015. PMID: 25959417 Free PMC article.
-
IL15RA is required for osteoblast function and bone mineralization.Bone. 2017 Oct;103:20-30. doi: 10.1016/j.bone.2017.06.003. Epub 2017 Jun 6. Bone. 2017. PMID: 28602725 Free PMC article.
-
The ecto-nucleotide pyrophosphatase/phosphodiesterase 2 promotes early osteoblast differentiation and mineralization in stromal stem cells.Am J Physiol Cell Physiol. 2024 Mar 1;326(3):C843-C849. doi: 10.1152/ajpcell.00692.2023. Epub 2024 Jan 15. Am J Physiol Cell Physiol. 2024. PMID: 38223929 Free PMC article.
-
Tissue-nonspecific alkaline phosphatase deficiency causes abnormal craniofacial bone development in the Alpl(-/-) mouse model of infantile hypophosphatasia.Bone. 2014 Oct;67:81-94. doi: 10.1016/j.bone.2014.06.040. Epub 2014 Jul 9. Bone. 2014. PMID: 25014884 Free PMC article.
References
-
- Terkeltaub R., Rosenbach M., Fong F., Goding J. (1994) Arthritis Rheum. 37, 934–941 - PubMed
-
- Johnson K., Moffa A., Chen Y., Pritzker K., Goding J., Terkeltaub R. (1999) J. Bone Miner. Res. 14, 883–892 - PubMed
-
- Johnson K., Vaingankar S., Chen Y., Moffa A., Goldring M. B., Sano K., Jin-Hua P., Sali A., Goding J., Terkeltaub R. (1999) Arthritis Rheum. 42, 1986–1997 - PubMed
-
- Johnson K. A., Hessle L., Vaingankar S., Wennerg C., Mauro S., Narisawa S., Goding J. W., Sano K., Millan J. L., Terkeltaub R. (2000) Am. J. Physiol. Int. Comp. Physiol. 279, R1365–R1377 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

