Molecular characterization of UpaB and UpaC, two new autotransporter proteins of uropathogenic Escherichia coli CFT073
- PMID: 21930758
- PMCID: PMC3255655
- DOI: 10.1128/IAI.05322-11
Molecular characterization of UpaB and UpaC, two new autotransporter proteins of uropathogenic Escherichia coli CFT073
Abstract
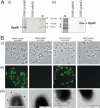
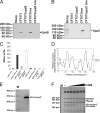
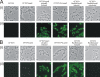
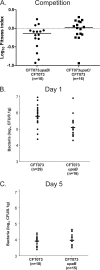
Uropathogenic Escherichia coli (UPEC) is the primary cause of urinary tract infection (UTI) in the developed world. The major factors associated with virulence of UPEC are fimbrial adhesins, which mediate specific attachment to host receptors and trigger innate host responses. Another group of adhesins is represented by the autotransporter (AT) subgroup of proteins. The genome-sequenced prototype UPEC strain CFT073 contains 11 putative AT-encoding genes. In this study, we have performed a detailed molecular characterization of two closely related AT adhesins from CFT073: UpaB (c0426) and UpaC (c0478). PCR screening revealed that the upaB and upaC AT-encoding genes are common in E. coli. The upaB and upaC genes were cloned and characterized in a recombinant E. coli K-12 strain background. This revealed that they encode proteins located at the cell surface but possess different functional properties: UpaB mediates adherence to several ECM proteins, while UpaC expression is associated with increased biofilm formation. In CFT073, upaB is expressed while upaC is transcriptionally repressed by the global regulator H-NS. In competitive colonization experiments employing the mouse UTI model, CFT073 significantly outcompeted its upaB (but not upaC) isogenic mutant strain in the bladder. This attenuated phenotype was also observed in single-challenge experiments, where deletion of the upaB gene in CFT073 significantly reduced early colonization of the bladder.
Figures

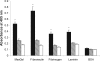
References
-
- Anderson GG, et al. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301: 105–107 - PubMed
-
- Beloin C, Dorman C. 2003. An extended role for the nucleoid structuring protein H-NS in the virulence gene regulatory cascade of Shigella flexneri. Mol. Microbiol. 47: 825–838 - PubMed
-
- Beloin C, et al. 2004. Global impact of mature biofilm lifestyle on Escherichia coli K-12 gene expression. Mol. Microbiol. 51: 659–674 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

