Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells
- PMID: 21930765
- PMCID: PMC3182064
- DOI: 10.1084/jem.20101159
Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells
Abstract
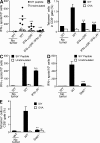
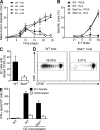
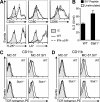
Despite lack of tumor control in many models, spontaneous T cell priming occurs frequently in response to a growing tumor. However, the innate immune mechanisms that promote natural antitumor T cell responses are undefined. In human metastatic melanoma, there was a correlation between a type I interferon (IFN) transcriptional profile and T cell markers in metastatic tumor tissue. In mice, IFN-β was produced by CD11c(+) cells after tumor implantation, and tumor-induced T cell priming was defective in mice lacking IFN-α/βR or Stat1. IFN signaling was required in the hematopoietic compartment at the level of host antigen-presenting cells, and selectively for intratumoral accumulation of CD8α(+) dendritic cells, which were demonstrated to be essential using Batf3(-/-) mice. Thus, host type I IFNs are critical for the innate immune recognition of a growing tumor through signaling on CD8α(+) DCs.
Figures
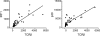

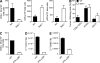
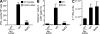
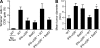
Comment in
-
Tumour immunology: IFNs boost cancer killers.Nat Rev Immunol. 2011 Oct 25;11(11):718. doi: 10.1038/nri3094. Nat Rev Immunol. 2011. PMID: 22025052 No abstract available.
References
-
- Anderson M.J., Shafer-Weaver K., Greenberg N.M., Hurwitz A.A. 2007. Tolerization of tumor-specific T cells despite efficient initial priming in a primary murine model of prostate cancer. J. Immunol. 178:1268–1276 - PubMed
-
- Anichini A., Molla A., Mortarini R., Tragni G., Bersani I., Di Nicola M., Gianni A.M., Pilotti S., Dunbar R., Cerundolo V., Parmiani G. 1999. An expanded peripheral T cell population to a cytotoxic T lymphocyte (CTL)–defined, melanocyte-specific antigen in metastatic melanoma patients impacts on generation of peptide-specific CTLs but does not overcome tumor escape from immune surveillance in metastatic lesions. J. Exp. Med. 190:651–667 10.1084/jem.190.5.651 - DOI - PMC - PubMed
-
- Basu S., Binder R.J., Suto R., Anderson K.M., Srivastava P.K. 2000. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int. Immunol. 12:1539–1546 10.1093/intimm/12.11.1539 - DOI - PubMed
-
- Belz G.T., Smith C.M., Eichner D., Shortman K., Karupiah G., Carbone F.R., Heath W.R. 2004. Cutting edge: conventional CD8 alpha+ dendritic cells are generally involved in priming CTL immunity to viruses. J. Immunol. 172:1996–2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

