Type I interferon is selectively required by dendritic cells for immune rejection of tumors
- PMID: 21930769
- PMCID: PMC3182061
- DOI: 10.1084/jem.20101158
Type I interferon is selectively required by dendritic cells for immune rejection of tumors
Abstract
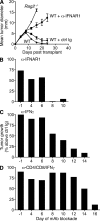
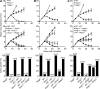
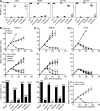
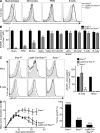
Cancer immunoediting is the process whereby the immune system suppresses neoplastic growth and shapes tumor immunogenicity. We previously reported that type I interferon (IFN-α/β) plays a central role in this process and that hematopoietic cells represent critical targets of type I IFN's actions. However, the specific cells affected by IFN-α/β and the functional processes that type I IFN induces remain undefined. Herein, we show that type I IFN is required to initiate the antitumor response and that its actions are temporally distinct from IFN-γ during cancer immunoediting. Using mixed bone marrow chimeric mice, we demonstrate that type I IFN sensitivity selectively within the innate immune compartment is essential for tumor-specific T cell priming and tumor elimination. We further show that mice lacking IFNAR1 (IFN-α/β receptor 1) in dendritic cells (DCs; Itgax-Cre(+)Ifnar1(f/f) mice) cannot reject highly immunogenic tumor cells and that CD8α(+) DCs from these mice display defects in antigen cross-presentation to CD8(+) T cells. In contrast, mice depleted of NK cells or mice that lack IFNAR1 in granulocytes and macrophage populations reject these tumors normally. Thus, DCs and specifically CD8α(+) DCs are functionally relevant targets of endogenous type I IFN during lymphocyte-mediated tumor rejection.
Figures






Comment in
-
Tumour immunology: IFNs boost cancer killers.Nat Rev Immunol. 2011 Oct 25;11(11):718. doi: 10.1038/nri3094. Nat Rev Immunol. 2011. PMID: 22025052 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

