GRK5 promotes F-actin bundling and targets bundles to membrane structures to control neuronal morphogenesis
- PMID: 21930777
- PMCID: PMC3207290
- DOI: 10.1083/jcb.201104114
GRK5 promotes F-actin bundling and targets bundles to membrane structures to control neuronal morphogenesis
Abstract
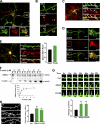
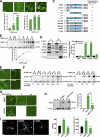
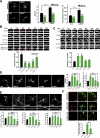
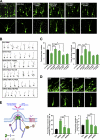
Neuronal morphogenesis requires extensive membrane remodeling and cytoskeleton dynamics. In this paper, we show that GRK5, a G protein-coupled receptor kinase, is critically involved in neurite outgrowth, dendrite branching, and spine morphogenesis through promotion of filopodial protrusion. Interestingly, GRK5 is not acting as a kinase but rather provides a key link between the plasma membrane and the actin cytoskeleton. GRK5 promoted filamentous actin (F-actin) bundling at the membranes of dynamic neuronal structures by interacting with both F-actin and phosphatidylinositol-4,5-bisphosphate. Moreover, separate domains of GRK5 mediated the coupling of actin cytoskeleton dynamics and membrane remodeling and were required for its effects on neuronal morphogenesis. Accordingly, GRK5 knockout mice exhibited immature spine morphology and deficient learning and memory. Our findings identify GRK5 as a critical mediator of dendritic development and suggest that coordinated actin cytoskeleton and membrane remodeling mediated by bifunctional actin-bundling and membrane-targeting molecules, such as GRK5, is crucial for proper neuronal morphogenesis and the establishment of functional neuronal circuitry.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

