Gata-3 negatively regulates the tumor-initiating capacity of mammary luminal progenitor cells and targets the putative tumor suppressor caspase-14
- PMID: 21930782
- PMCID: PMC3209243
- DOI: 10.1128/MCB.05766-11
Gata-3 negatively regulates the tumor-initiating capacity of mammary luminal progenitor cells and targets the putative tumor suppressor caspase-14
Abstract
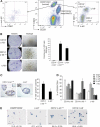
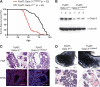
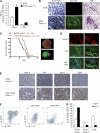
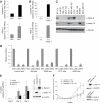
The transcription factor Gata-3 is a definitive marker of luminal breast cancers and a key regulator of mammary morphogenesis. Here we have explored a role for Gata-3 in tumor initiation and the underlying cellular mechanisms using a mouse model of "luminal-like" cancer. Loss of a single Gata-3 allele markedly accelerated tumor progression in mice carrying the mouse mammary tumor virus promoter-driven polyomavirus middle T antigen (MMTV-PyMT mice), while overexpression of Gata-3 curtailed tumorigenesis. Through the identification of two distinct luminal progenitor cells in the mammary gland, we demonstrate that Gata-3 haplo-insufficiency increases the tumor-initiating capacity of these progenitors but not the stem cell-enriched population. Overexpression of a conditional Gata-3 transgene in the PyMT model promoted cellular differentiation and led to reduced tumor-initiating capacity as well as diminished angiogenesis. Transcript profiling studies identified caspase-14 as a novel downstream target of Gata-3, in keeping with its roles in differentiation and tumorigenesis. A strong association was evident between GATA-3 and caspase-14 expression in preinvasive ductal carcinoma in situ samples, where GATA-3 also displayed prognostic significance. Overall, these studies identify GATA-3 as an important regulator of tumor initiation through its ability to promote the differentiation of committed luminal progenitor cells.
Figures




References
-
- Arnold J. M., et al. 2010. Frequent somatic mutations of GATA3 in non-BRCA1/BRCA2 familial breast tumors, but not in BRCA1-, BRCA2- or sporadic breast tumors. Breast Cancer Res. Treat. 119:491–496 - PubMed
-
- Asselin-Labat M. L., et al. 2007. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat. Cell Biol. 9:201–209 - PubMed
-
- Bertucci F., et al. 2000. Gene expression profiling of primary breast carcinomas using arrays of candidate genes. Hum. Mol. Genet. 9:2981–2991 - PubMed
-
- Bouras T., et al. 2008. Notch signaling regulates mammary stem cell function and luminal cell-fate commitment. Cell Stem Cell 3:429–441 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
