Distinct functions of Sox2 control self-renewal and differentiation in the osteoblast lineage
- PMID: 21930787
- PMCID: PMC3209254
- DOI: 10.1128/MCB.05798-11
Distinct functions of Sox2 control self-renewal and differentiation in the osteoblast lineage
Abstract
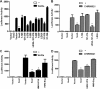
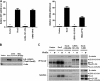
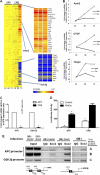
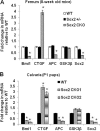
The transcription factor Sox2 is a key player in the maintenance of pluripotency and "stemness." We have previously shown that Sox2 maintains self-renewal in the osteoblast lineage while inhibiting differentiation (U. Basu-Roy et al., Cell Death Differ. 17:1345-1353, 2010; A. Mansukhani, D. Ambrosetti, G. Holmes, L. Cornivelli, and C. Basilico, J. Cell Biol. 168:1065-1076, 2005). Sox2 also interferes with Wnt signaling by binding β-catenin, a central mediator of the Wnt pathway. Here we show that these multiple functions of Sox2 are encoded in distinct domains. The self-renewal function of Sox2 is dependent on its transcriptional activity and requires both its DNA-binding and C-terminal activation regions, while only the third C-terminal transactivation (TA) region is required for binding β-catenin and interfering with Wnt-induced transcription. The results of gene expression analysis upon Sox2 deletion strongly support the notion that Sox2 maintains stemness. We show also that Sox2 suppresses differentiation by attenuating Wnt signaling by posttranscriptional and transcriptional mechanisms and that adenomatous polyposis coli (APC) and GSK3β, which are negative regulators of the Wnt pathway, are direct Sox2 targets in osteoblasts. Several genes, such as the FoxP1 and BMI-1 genes, that are associated with stemness are downregulated upon Sox2 inactivation. Constitutive expression of the Polycomb complex member BMI-1 can bypass the Sox2 requirement for self-renewal but does not affect differentiation. Our results establish a connection between Sox2 and BMI-1 in maintaining self-renewal and identify BMI-1 as a key mediator of Sox2 function.
Figures




References
-
- Ambrosetti D. C., Scholer H. R., Dailey L., Basilico C. 2000. Modulation of the activity of multiple transcriptional activation domains by the DNA binding domains mediates the synergistic action of Sox2 and Oct-3 on the fibroblast growth factor-4 enhancer. J. Biol. Chem. 275:23387–23397 - PubMed
-
- Aubin J., Triffit J. 2002. Mesenchymal cells and osteoblast differentiation, p. 59–82 In Bilezikian J., Raisz L. G., Rodan G. A.(ed.), Principles of bone biology, vol. 1 Academic Press, New York, NY
-
- Baron R., Rawadi G. 2007. Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology 148:2635–2643 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
