Coincident light and clock regulation of pseudoresponse regulator protein 37 (PRR37) controls photoperiodic flowering in sorghum
- PMID: 21930910
- PMCID: PMC3182727
- DOI: 10.1073/pnas.1106212108
Coincident light and clock regulation of pseudoresponse regulator protein 37 (PRR37) controls photoperiodic flowering in sorghum
Abstract
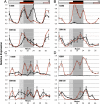
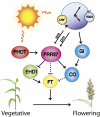
Optimal flowering time is critical to the success of modern agriculture. Sorghum is a short-day tropical species that exhibits substantial photoperiod sensitivity and delayed flowering in long days. Genotypes with reduced photoperiod sensitivity enabled sorghum's utilization as a grain crop in temperate zones worldwide. In the present study, Ma(1), the major repressor of sorghum flowering in long days, was identified as the pseudoresponse regulator protein 37 (PRR37) through positional cloning and analysis of SbPRR37 alleles that modulate flowering time in grain and energy sorghum. Several allelic variants of SbPRR37 were identified in early flowering grain sorghum germplasm that contain unique loss-of-function mutations. We show that in long days SbPRR37 activates expression of the floral inhibitor CONSTANS and represses expression of the floral activators Early Heading Date 1, FLOWERING LOCUS T, Zea mays CENTRORADIALIS 8, and floral induction. Expression of SbPRR37 is light dependent and regulated by the circadian clock, with peaks of RNA abundance in the morning and evening in long days. In short days, the evening-phase expression of SbPRR37 does not occur due to darkness, allowing sorghum to flower in this photoperiod. This study provides insight into an external coincidence mechanism of photoperiodic regulation of flowering time mediated by PRR37 in the short-day grass sorghum and identifies important alleles of SbPRR37 that are critical for the utilization of this tropical grass in temperate zone grain and bioenergy production.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
CONSTANS is a photoperiod regulated activator of flowering in sorghum.BMC Plant Biol. 2014 May 28;14:148. doi: 10.1186/1471-2229-14-148. BMC Plant Biol. 2014. PMID: 24884377 Free PMC article.
-
Sorghum phytochrome B inhibits flowering in long days by activating expression of SbPRR37 and SbGHD7, repressors of SbEHD1, SbCN8 and SbCN12.PLoS One. 2014 Aug 14;9(8):e105352. doi: 10.1371/journal.pone.0105352. eCollection 2014. PLoS One. 2014. PMID: 25122453 Free PMC article.
-
Allelic variants in the PRR37 gene and the human-mediated dispersal and diversification of sorghum.Theor Appl Genet. 2015 Sep;128(9):1669-83. doi: 10.1007/s00122-015-2523-z. Epub 2015 May 16. Theor Appl Genet. 2015. PMID: 25982128
-
Photoperiodic flowering: time measurement mechanisms in leaves.Annu Rev Plant Biol. 2015;66:441-64. doi: 10.1146/annurev-arplant-043014-115555. Epub 2014 Dec 12. Annu Rev Plant Biol. 2015. PMID: 25534513 Free PMC article. Review.
-
Energy sorghum--a genetic model for the design of C4 grass bioenergy crops.J Exp Bot. 2014 Jul;65(13):3479-89. doi: 10.1093/jxb/eru229. Epub 2014 Jun 22. J Exp Bot. 2014. PMID: 24958898 Review.
Cited by
-
A Pleiotropic Flowering Time QTL Exhibits Gene-by-Environment Interaction for Fitness in a Perennial Grass.Mol Biol Evol. 2022 Oct 7;39(10):msac203. doi: 10.1093/molbev/msac203. Mol Biol Evol. 2022. PMID: 36149808 Free PMC article.
-
Sugars, the clock and transition to flowering.Front Plant Sci. 2013 Feb 14;4:22. doi: 10.3389/fpls.2013.00022. eCollection 2013. Front Plant Sci. 2013. PMID: 23420760 Free PMC article.
-
Stability and genetic control of morphological, biomass and biofuel traits under temperate maritime and continental conditions in sweet sorghum (Sorghum bicolour).Theor Appl Genet. 2015 Sep;128(9):1685-701. doi: 10.1007/s00122-015-2538-5. Epub 2015 May 16. Theor Appl Genet. 2015. PMID: 25982132
-
Loss-of-Function Alleles of Heading date 1 (Hd1) Are Associated With Adaptation of Temperate Japonica Rice Plants to the Tropical Region.Front Plant Sci. 2018 Dec 10;9:1827. doi: 10.3389/fpls.2018.01827. eCollection 2018. Front Plant Sci. 2018. PMID: 30619400 Free PMC article.
-
Ghd8 controls rice photoperiod sensitivity by forming a complex that interacts with Ghd7.BMC Plant Biol. 2019 Nov 1;19(1):462. doi: 10.1186/s12870-019-2053-y. BMC Plant Biol. 2019. PMID: 31675987 Free PMC article.
References
-
- Smith CW, Frederiksen RA. In: Sorghum: Origin, History, Technology, and Production. Smith CW, Frederikson RA, editors. New York: John Wiley & Sons; 2000. pp. 191–223.
-
- Rooney WL, Blumenthal J, Bean B, Mullet JE. Designing sorghum as a dedicated bioenergy feedstock. Biofuel Bioprod Bior. 2007;1:147–157.
-
- Garner WW, Allard HA. Effect of the relative length of day and night and other factors of the environment on growth and reproduction in plants. J Agric Res. 1920;18:553–606.
-
- Quinby JR. Sorghum Improvement and the Genetics of Growth. College Station, TX: Texas A&M University Press; 1974.
-
- Bünning E. Circadian rhythms and the time measurement in photoperiodism. Cold Spring Harbor Symp Quant Biol. 1960;25:249–256. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

