Integration of low temperature and light signaling during cold acclimation response in Arabidopsis
- PMID: 21930922
- PMCID: PMC3182711
- DOI: 10.1073/pnas.1107161108
Integration of low temperature and light signaling during cold acclimation response in Arabidopsis
Abstract
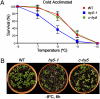
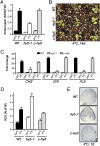
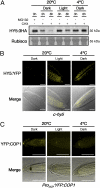
Certain plants increase their freezing tolerance in response to low nonfreezing temperatures, an adaptive process named cold acclimation. Light has been shown to be required for full cold acclimation, although how light and cold signals integrate and cross-talk to enhance freezing tolerance still remains poorly understood. Here, we show that HY5 levels are regulated by low temperature transcriptionally, via a CBF- and ABA-independent pathway, and posttranslationally, via protein stabilization through nuclear depletion of COP1. Furthermore, we demonstrate that HY5 positively regulates cold-induced gene expression through the Z-box and other cis-acting elements, ensuring the complete development of cold acclimation. These findings uncover unexpected functions for HY5, COP1, and the Z-box in Arabidopsis response to low temperature, provide insights on how cold and light signals integrate to optimize plant survival under freezing temperatures, and reveal the complexity of the molecular mechanisms plants have evolved to respond and adapt to their fluctuating natural environment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
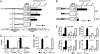


References
-
- Levitt J. Responses of Plants to Environmental Stresses: Chilling, Freezing and High Temperatures Stresses. New York: Academic; 1980.
-
- Olsen JE, et al. Ectopic expression of phytochrome A in hybrid aspen changes critical daylength for growth and prevents cold acclimation. Plant J. 1997;12:1339–1350.
-
- Kim HJ, Kim YK, Park JY, Kim J. Light signalling mediated by phytochrome plays an important role in cold-induced gene expression through the C-repeat/dehydration responsive element (C/DRE) in Arabidopsis thaliana. Plant J. 2002;29:693–704. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

