Nucleosides from Phlebotomus papatasi salivary gland ameliorate murine collagen-induced arthritis by impairing dendritic cell functions
- PMID: 21930966
- PMCID: PMC3195336
- DOI: 10.4049/jimmunol.1003404
Nucleosides from Phlebotomus papatasi salivary gland ameliorate murine collagen-induced arthritis by impairing dendritic cell functions
Abstract
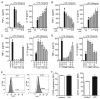
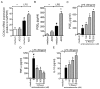
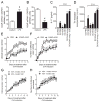
Among several pharmacological compounds, Phlebotomine saliva contains substances with anti-inflammatory properties. In this article, we demonstrated the therapeutic activity of salivary gland extract (SGE) of Phlebotomus papatasi in an experimental model of arthritis (collagen-induced arthritis [CIA]) and identified the constituents responsible for such activity. Daily administration of SGE, initiated at disease onset, attenuated the severity of CIA, reducing the joint lesion and proinflammatory cytokine release. In vitro incubation of dendritic cells (DCs) with SGE limited specific CD4(+) Th17 cell response. We identified adenosine (ADO) and 5'AMP as the major salivary molecules responsible for anti-inflammatory activities. Pharmacologic inhibition of ADO A2(A) receptor or enzymatic catabolism of salivary nucleosides reversed the SGE-induced immunosuppressive effect. Importantly, CD73 (ecto-5'-nucleotidase enzyme) is expressed on DC surface during stage of activation, suggesting that ADO is also generated by 5'AMP metabolism. Moreover, both nucleosides mimicked SGE-induced anti-inflammatory activity upon DC function in vitro and attenuated establishment of CIA in vivo. We reveal that ADO and 5'AMP are present in pharmacological amounts in P. papatasi saliva and act preferentially on DC function, consequently reducing Th17 subset activation and suppressing the autoimmune response. Thus, it is plausible that these constituents might be promising therapeutic molecules to target immune inflammatory diseases.
Conflict of interest statement
DISCLOSURES: The authors have no conflicting financial interests
Figures





References
-
- Ribeiro JM. Role of saliva in blood-feeding by arthropods. Annual review of entomology. 1987;32:463–478. - PubMed
-
- Ribeiro JM, I, Francischetti M. Role of arthropod saliva in blood feeding: sialome and post-sialome perspectives. Annual review of entomology. 2003;48:73–88. - PubMed
-
- Ribeiro JM, Weis JJ, Telford SR., 3rd Saliva of the tick Ixodes dammini inhibits neutrophil function. Experimental parasitology. 1990;70:382–388. - PubMed
-
- Warburg A, Schlein Y. The effect of post-bloodmeal nutrition of Phlebotomus papatasi on the transmission of Leishmania major. The American journal of tropical medicine and hygiene. 1986;35:926–930. - PubMed
-
- Donnelly KB, Lima HC, Titus RG. Histologic characterization of experimental cutaneous leishmaniasis in mice infected with Leishmania braziliensis in the presence or absence of sand fly vector salivary gland lysate. The Journal of parasitology. 1998;84:97–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

