Adipose tissue ATP binding cassette transporter A1 contributes to high-density lipoprotein biogenesis in vivo
- PMID: 21931081
- PMCID: PMC3202242
- DOI: 10.1161/CIRCULATIONAHA.111.025445
Adipose tissue ATP binding cassette transporter A1 contributes to high-density lipoprotein biogenesis in vivo
Abstract
Background: Adipose tissue (AT) is the body's largest free cholesterol reservoir and abundantly expresses ATP binding cassette transporter A1 (ABCA1), a key cholesterol transporter for high-density lipoprotein (HDL) biogenesis. However, the extent to which AT ABCA1 expression contributes to HDL biogenesis in vivo is unknown.
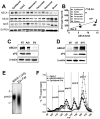
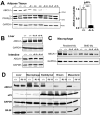
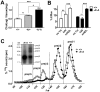
Methods and results: Adipocyte-specific ABCA1 knockout mice (ABCA1(-A/-A)) were generated by crossing ABCA1(floxed) mice with aP2Cre transgenic mice. AT from ABCA1(-A/-A) mice had <10% of wild-type ABCA1 protein expression but normal hepatic and intestinal expression. Deletion of adipocyte ABCA1 resulted in a significant decrease in plasma HDL cholesterol (approximately 15%) and apolipoprotein A-I (approximately 13%) concentrations. AT from ABCA1(-A/-A) mice had a 2-fold increase in free cholesterol content compared with wild-type mice and failed to efflux cholesterol to apolipoprotein A-I. However, cholesterol efflux from AT to plasma HDL was similar for both genotypes of mice. Incubation of wild-type AT explants with apolipoprotein A-I resulted in the formation of multiple discrete-sized nascent HDL particles ranging in diameter from 7.1 to 12 nm; similar incubations with ABCA1(-A/-A) AT explants resulted in nascent HDL <8 nm. Plasma decay and tissue uptake of wild-type (125)I-HDL tracer were similar in both genotypes of recipient mice, suggesting that adipocyte ABCA1 deficiency reduces plasma HDL concentrations solely by reducing nascent HDL particle formation.
Conclusions: We provide in vivo evidence that AT ABCA1-dependent cholesterol efflux and nascent HDL particle formation contribute to systemic HDL biogenesis and that AT ABCA1 expression plays an important role in adipocyte cholesterol homeostasis.
Figures


References
-
- Durnin JV, Rahaman MM. The assessment of the amount of fat in the human body from measurements of skinfold thickness. Br J Nutr. 1967;21:681–689. - PubMed
-
- Krause BR, Hartman AD. Adipose tissue and cholesterol metabolism. J Lipid Res. 1984;25:97–110. - PubMed
-
- Prattes S, Horl G, Hammer A, Blaschitz A, Graier WF, Sattler W, Zechner R, Steyrer E. Intracellular distribution and mobilization of unesterified cholesterol in adipocytes: triglyceride droplets are surrounded by cholesterol-rich ER-like surface layer structures. J Cell Sci. 2000;113 ( Pt 17):2977–2989. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

