Histopathology and functional correlations in a patient with a mutation in RPE65, the gene for retinol isomerase
- PMID: 21931134
- PMCID: PMC3208160
- DOI: 10.1167/iovs.11-7973
Histopathology and functional correlations in a patient with a mutation in RPE65, the gene for retinol isomerase
Abstract
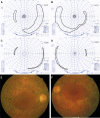
Purpose: Here the authors describe the structural features of the retina and retinal pigment epithelium (RPE) in postmortem donor eyes of a 56-year-old patient with a homozygous missense RPE65 mutation (Ala132Thr) and correlate the pathology with the patient's visual function last measured at age 51.
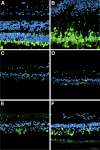
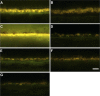
Methods: Eyes were enucleated within 13.5 hours after death. Representative areas from the macula and periphery were processed for light and electron microscopy. Immunofluorescence was used to localize the distribution of RPE65, rhodopsin, and cone arrestin. The autofluorescence in the RPE was compared with that of two normal eyes from age-similar donors.
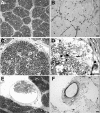
Results: Histologic examination revealed the loss of rods and cones across most areas of the retina, attenuated retinal vessels, and RPE thinning in both eyes. A small number of highly disorganized cones were present in the macula that showed simultaneous labeling with cone arrestin and red/green or blue opsin. RPE65 immunoreactivity and RPE autofluorescence were reduced compared with control eyes in all areas studied. Rhodopsin labeling was observed in rods in the far periphery. The optic nerve showed a reduced number of axons.
Conclusions: The clinical findings of reduced visual acuity, constricted fields, and reduced electroretinograms (ERGs) 5 years before death correlated with the small number of cones present in the macula and the extensive loss of photoreceptors in the periphery. The absence of autofluorescence in the RPE suggests that photoreceptor cells were probably missing across the retina for extended periods of time. Possible mechanisms that could lead to photoreceptor cell death are discussed.
Figures








Similar articles
-
Ciliary neurotrophic factor (CNTF) protects retinal cone and rod photoreceptors by suppressing excessive formation of the visual pigments.J Biol Chem. 2018 Sep 28;293(39):15256-15268. doi: 10.1074/jbc.RA118.004008. Epub 2018 Aug 16. J Biol Chem. 2018. PMID: 30115683 Free PMC article.
-
Dominant late-onset retinal degeneration with regional variation of sub-retinal pigment epithelium deposits, retinal function, and photoreceptor degeneration.Ophthalmology. 2000 Dec;107(12):2256-66. doi: 10.1016/s0161-6420(00)00419-x. Ophthalmology. 2000. PMID: 11097607
-
A comprehensive clinical and biochemical functional study of a novel RPE65 hypomorphic mutation.Invest Ophthalmol Vis Sci. 2008 Dec;49(12):5235-42. doi: 10.1167/iovs.07-1671. Epub 2008 Jul 3. Invest Ophthalmol Vis Sci. 2008. PMID: 18599565 Free PMC article.
-
Retinal pigment epithelium 65 kDa protein (RPE65): An update.Prog Retin Eye Res. 2022 May;88:101013. doi: 10.1016/j.preteyeres.2021.101013. Epub 2021 Oct 2. Prog Retin Eye Res. 2022. PMID: 34607013 Free PMC article. Review.
-
Key enzymes of the retinoid (visual) cycle in vertebrate retina.Biochim Biophys Acta. 2012 Jan;1821(1):137-51. doi: 10.1016/j.bbalip.2011.03.005. Epub 2011 Apr 5. Biochim Biophys Acta. 2012. PMID: 21447403 Free PMC article. Review.
Cited by
-
Alu complementary DNA is enriched in atrophic macular degeneration and triggers retinal pigmented epithelium toxicity via cytosolic innate immunity.Sci Adv. 2021 Oct;7(40):eabj3658. doi: 10.1126/sciadv.abj3658. Epub 2021 Sep 29. Sci Adv. 2021. PMID: 34586848 Free PMC article.
-
Mutational screening of LCA genes emphasizing RPE65 in South Indian cohort of patients.PLoS One. 2013 Sep 16;8(9):e73172. doi: 10.1371/journal.pone.0073172. eCollection 2013. PLoS One. 2013. PMID: 24066033 Free PMC article.
-
Increased levels of gene therapy may not be beneficial in retinal disease.Proc Natl Acad Sci U S A. 2013 May 7;110(19):E1705. doi: 10.1073/pnas.1303746110. Epub 2013 Apr 3. Proc Natl Acad Sci U S A. 2013. PMID: 23553840 Free PMC article. No abstract available.
-
Histopathology of the Retina from a Three Year-Old Suspected to Have Joubert Syndrome.Austin J Clin Ophthalmol. 2015;2(4):1057. Epub 2015 Sep 21. Austin J Clin Ophthalmol. 2015. PMID: 27747301 Free PMC article.
-
Clinical Perspective: Treating RPE65-Associated Retinal Dystrophy.Mol Ther. 2021 Feb 3;29(2):442-463. doi: 10.1016/j.ymthe.2020.11.029. Epub 2020 Dec 3. Mol Ther. 2021. PMID: 33278565 Free PMC article. Review.
References
-
- Cremers FP, van den Hurk JA, den Hollander AI. Molecular genetics of Leber congenital amaurosis. Hum Mol Genet. 2002;11:1169–1176 - PubMed
-
- den Hollander AI, Roepman R, Koenekoop RK, Cremers FP. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog Retin Eye Res. 2008;27:391–419 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

